|
Back to Fall Congress
Obstruction and overdistension-mediated bladder injury results in urothelial dedifferentiation marked by decreased Foxa1 and uroplakin expression and expansion of basal urothelium
Christina B. Ching, MD, Magdalena M. Grabowska, PhD, Douglas W. Strand, PhD, Heidi A. Stephany, MD, Stacy T. Tanaka, MD, John C. Thomas, MD, John C. Pope, IV, MD, Mark C. Adams, MD, John W. Brock, III, MD, Simon W. Hayward, PhD, Robert J. Matusik, PhD, David J. DeGraff, PhD, Douglass B. Clayton, MD.
Vanderbilt University, Nashville, TN, USA.
Background:
Cycles of bladder injury occur in various diseases of pediatric urology, including intermittent to chronic bladder obstruction. Specific alterations in urothelial cell biology in response to injury are not fully characterized. Understanding how the cellular biology of the urothelium responds to such injury will enable us to understand how injuries result in a disruption of normal bladder healing. We hypothesized that alterations in cellular differentiation status are implicated in urothelial regeneration following bladder injury.
Methods:
Ovariectomized female C57/BL6 mice underwent one of two models of benign bladder injury either by partial bladder outlet obstruction (pBOO) or bladder overdistension. pBOO mice underwent partial ligation of the urethra with silk suture while bladder overdistension animals were catheterized and distended for 90 minutes at 60 cm H20 using 0.9% saline. Mice were sacrificed after pBOO at weeks 2, 4, and 13, and at 6 months. Two overdistension groups were utilized: acute and chronic. Animals in the acute group underwent a single distension for 90 minutes and were sacrifice at 0, 3, 6, 12, 24, 48, or 72 hours following relief of distension. In the chronic group, animals underwent a single distension daily for 5 consecutive days and were sacrificed immediately after the last distension. Bladder tissue was harvested, formalin fixed, and paraffin embedded. Standard H&E staining was performed to assess gross histological changes, as well as determine the extent of inflammatory infiltrate. In addition, immunofluorescence was performed to assess levels of markers of urothelial proliferation (Ki-67), apoptosis (caspase-3), and differentiation (cytokeratins 5, 14 [CK5/CK14], uroplakin 3 [UP3], and Foxa1).
Results:
By H&E, both acute and chronic bladder distension stimulates an intense inflammatory response which follows a distinct temporal pattern. Similarly, intense inflammation is also present in pBOO samples. Urothelial response to distension injury is primarily proliferative beginning 24 hours after acute distension and is localized to the basal layer of the urothelium in both injury models. This proliferative phenotype is maintained following chronic distension and pBOO. In both injury models, the urothelium responds by demonstrating an expansion of CK14+ basal cells. These CK14+ cells express heterogenous Foxa1 compared to more superficially located CK14- cells. Further evidence of urothelial dedifferentiation is seen in animals following pBOO where we see expansion of CK5+ cells coupled with loss of normal UP3 expression (FIGURE).
Conclusions:
The response to pBOO and overdistension includes increased urothelial proliferation and expression of markers of basal uroepithelium. These observations, in conjunction with the decreased expression of markers of terminal differentiation suggest the existence of a programmed wave of dedifferentiation followed by benign injury.
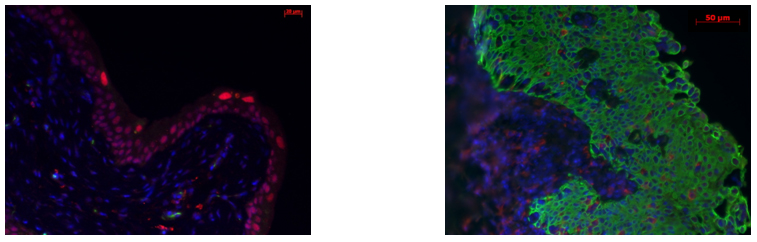
Control mouse (left) demonstrating umbrella cell UP3+ staining (cytoplasm red) and basal/intermediate layer CK5+ staining (membrane green). UP3 expression is lost at 4 weeks pBOO (right) with expansion of CK5+ cells to the superficial/umbrella cell layer. 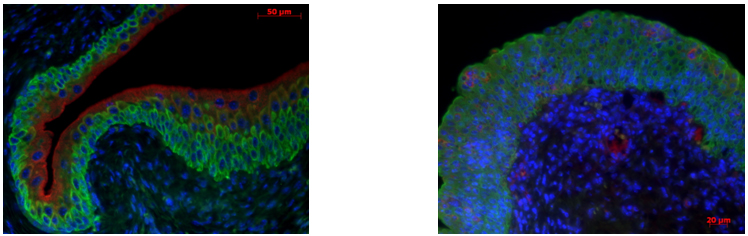
Control mouse (left) demonstrating absence of CK14+ staining (membrane green). Expansion of CK14+ staining in the 13 week pBOO (right). FOXA1 (nucleus red) staining is decreased in CK14+ cells.
Back to Fall Congress
|



