Systematic Review of Bladder Cancer Outcomes in Patients with Spina Bifida
Kyle O. Rove, MD1, Douglas A. Husmann, MD2, Duncan T. Wilcox, MBBS MD3, Ty T. Higuchi, MD, PhD1.
1University of Colorado, Aurora, CO, USA, 2Mayo Clinic, Rochester, MN, USA, 3Children's Hospital Colorado, Aurora, CO, USA.
BACKGROUND: Bladder cancer is a devastating diagnosis in patients with spina bifida, often presenting at high stage with high mortality, regardless of interventions. Some have reported bladder augmentation an independent risk factor for development of cancer. Neurogenic bladder unto itself may be a risk factor. We reviewed existing literature for cases of bladder cancer in spina bifida patients to analyze risk factors and to determine patient outcomes.
METHODS: A systematic literature search using PubMed was conducted by cross referencing the terms “spina bifida,” “myelomeningocele,” “spina dysraphism,” “cystoplasty,” “ileocystoplasty,” “gastric cystoplasty,” “augmentation,” and “neurogenic bladder” with “bladder cancer” and “bladder carcinoma.” Studies were selected for inclusion according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Inclusion criteria were studies with patients that had an underlying diagnosis of spina bifida and bladder cancer with data available on age, stage and mortality status. Studies were excluded if patients had a congenital bladder anomaly other than spina bifida, spina cord injury, history of tuberculosis or schistosomiasis, or prior ureterosigmoidostomy. We hypothesized there would be no difference in overall survival (OS) between patients with and without prior bladder augmentation. Secondary outcomes of interest were differences in OS between gender, age, whether patients were on a regular cystoscopic surveillance program, stage and type of bladder augmentation. For time-dependent analyses, log-rank analysis was used, and patients were censored if still alive at last follow up. Univariate and multivariate analyses using Cox proportional hazards models were performed.
RESULTS: 51 patients were identified from 28 studies with a median age at bladder cancer diagnosis of 41 years (range 13-73). Median follow up was 6 months (range 0-144 months). 1- and 2-year OS for all patients was 46.7% and 32.4%, respectively. 18 (35%) were on a surveillance program. 35 (68%) presented with AJCC stage III or IV bladder cancer. Two-year OS was significantly different between those with (70.1%) and without augmentation (14.5%, p=0.017), thus rejecting our primary hypothesis. Differences in OS by stage, gender, management with intermittent catheterization, or use of surveillance cystoscopic program were not significant on univariate analysis, while only augmentation status remained a significant predictor of OS in a multivariate model (HR 0.232, 95% CI 0.067-0.797, p=0.020). No significant differences in OS were seen between groups based on management with long-term indwelling catheter, diversion with ileal conduit, age, or histopathology. Secondary analysis was performed removing patients with gastric augmentation (n=8), and no difference in OS was seen between patients with (n=8) and without augmentation (n=35, p=0.217).
CONCLUSIONS: Patients with spina bifida who develop bladder cancer have aggressive disease. Bladder augmentation does not appear to be an independent risk factor for bladder cancer. Regular clinical follow up is paramount. Risk of bladder cancer should be discussed with all spina bifida patients as they graduate from pediatric urology care. 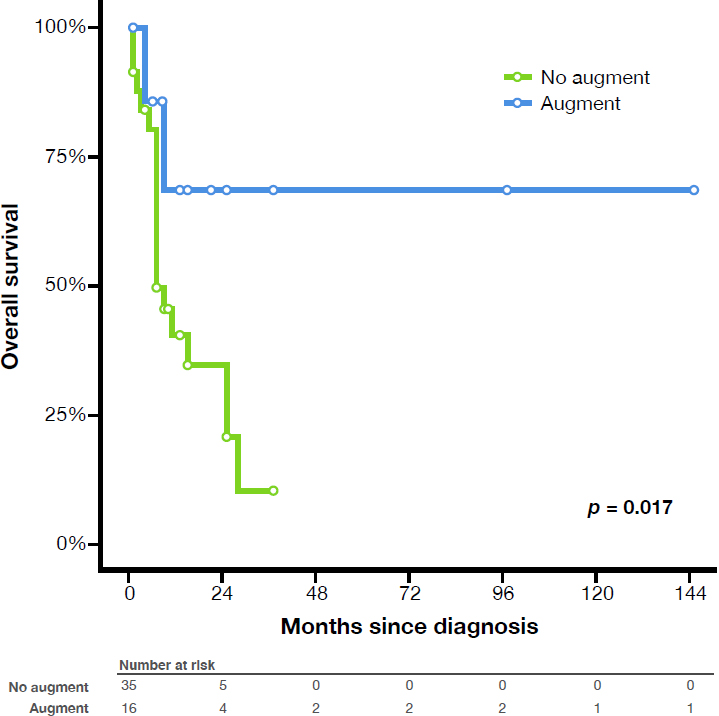
Back to 2016 Fall Congress
