Androgen Receptor Activity Modulates Bladder Hypertrophy in Partial Outlet Obstruction
Diana K. Bowen, MD, Paula Firmiss, BA, Natalie Kulkulka, MD, Robert Dettman, PhD, Edward M. Gong, MD.
Ann & Robert H. Lurie Childrens Hospital of Chicago, Chicago, IL, USA.
BACKGROUND: Previously our lab has demonstrated in a mouse model the negative effects of testosterone (T) on bladder remodeling in the setting of partial bladder outlet obstruction (PO), as evidenced by increased bladder weight and collagen deposition. Our hypothesis was that these androgen effects are mediated through the androgen receptor (AR). The objective of this study was to test if PO alters the AR, define its pathway in the obstructed mouse bladder, and the subsequent downstream effects.
METHODS: 8-10 week old male CD-1 mice underwent partial obstruction surgery at the bladder neck, with or without castration by bilateral orchiectomy. In a subset of the castrate PO mice (CPO), either a dihydrotestosterone (DHT) continuous release pellet was inserted subcutaneously (CPOD), or testosterone cypionate was injected subcutaneously (CPOT) at the time of surgery and then weekly thereafter. Functional evaluation was conducted with voided stain on paper at days 1, 7 and 28. Serum hormone levels including T, DHT, and Estradiol (E2) were drawn at the time of sacrifice. Whole bladders were analyzed at day 28 by weight (ratio of bladder to body) and immunofluorescence (IF) antibody staining of nuclear versus cytoplasmic AR. Quantitative PCR (qPCR) was performed at different time points to determine levels of gene expression. A real time (RT)-PCR microarray analysis of AR-regulated genes was performed in sham and PO animals. Microarray findings were validated by qPCR, western blot, and IF staining of bladder sections.
RESULTS: Mean bladder weight was highest in the CPOD group (4.2 mg/g ± SEM 1.7 vs. Sham 0.96 ± 0.2, CPOT 2.3 ± 0.5). There was no significant difference in serum E2 level across groups. Collagen 1a1 IF staining was increased in CPODs (27.2% ± SEM 1.9 vs. Sham 9.3 ± 2.7, PO 17.5 ± 2.5, CPO 8.3 ± 2.5, CPOT 19.3 ± 4.9). AR staining demonstrated increased expression and nuclear localization in CPODs (Figure 1). At 7 days AR gene expression was upregulated in CPOT and CPOD groups when compared to CPO, and normalized by 28 days. AR microarray RT-PCR revealed transcription factor Nkx3.1 was upregulated 2.5 times in PO normalized to Sham. This was validated by qPCR and western. IF revealed that nuclear Nkx3.1 was present in stromal cells of PO bladders.
CONCLUSIONS: Our findings indicate that testosterone plays a central role in bladder fibrosis following PO in male mice. Furthermore AR is active in bladder cells and mediates the production of Nkx3.1, a transcription factor thought to be exclusively expressed in the prostate. Interestingly, the site of expression of Nkx3.1 is in the stromal compartment, the site where we recently showed contains bladder intrinsic mesenchymal stem cells (MSCs). Thus, we hypothesize that dysregulation of Nkx3.1, downstream of testosterone and AR in bladder MSCs, may play an important role in the genesis of fibrosis following PO. 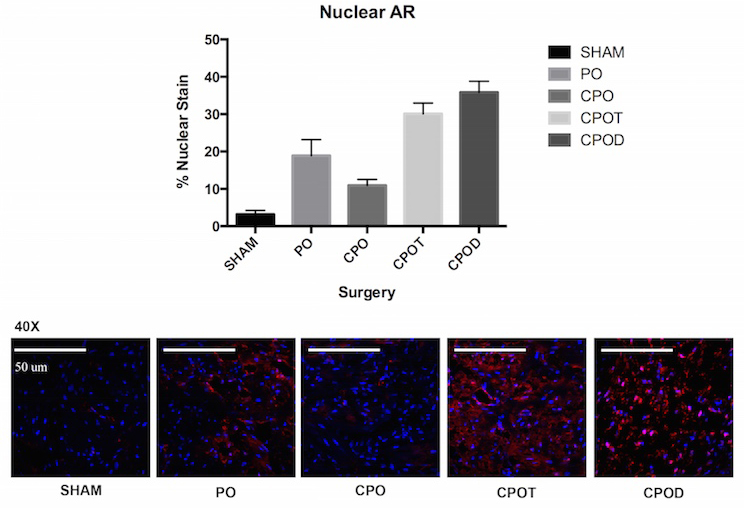
Back to 2016 Fall Congress
