KCTD13 gene dosage changes result in GU anomalies via dysregulation of androgen receptor signalling.
Abhishek Seth, MD, In Seon Choi, PhD, Armando Rivera, PhD, Shayne Lewis, PhD, Carolina Jorgez, PhD, Dolores J. Lamb, PhD.
Baylor College of Medicine, Houston, TX, USA.
Objective: The molecular basis for hypospadias and lower urogenital defects is poorly understood. Using comparative genomic hybridization arrays (aCGH), we identified a novel candidate gene, potassium channel tetramerization domain containing 13, KCTD 13 at 16p11.2 in patients with hypospadias, and cryptorchidism. KCDT13 encodes a substrate-specific adapter of a BCR E3 ubiquitin-protein ligase complex, which regulates the cytoskeleton and cell migration via ubquitination of RHOA. E3 ligases also differentially regulate androgen receptor (AR) signaling. Given its role in mediating cellular processes and AR pathway, we hypothesize that defect in KCTD13 results in androgen receptor dysregulation leading to GU tract anomalies.
Methods: Genomic DNA from pediatric patients with hypospadias, cryptorchidism, ambiguous genitalia and control patients was analyzed by aCGH CRISPR genome editing was used to generate and evaluate phenotype of Kctd13 null mice. AR signaling was studied using in vitro cultures of AR-HeLa cells with and without knockdown (KS) of KCTD13.
Results: Two patients with CNVs in KCTD13 and GU anomalies (hypospadias and cryptorchidism) were identified using aCGH. We identified 27 other patients in the literature and public databases (DECIPHER) as well as 5 additional patients in our cohort with defects in KCTD13 and concomitant GU anomalies, ranging from hypospadias, cryptorchidism, VUR and UPJO. AR levels increased with KCTD13 knockdown (KD) in cultures of AR transfected HeLa cells. Quantitative RT-PCR results showed that mRNA levels of AR upon KCTD13 KD were comparable to the control in both LNCaP cells and HeLa-AR cells, indicating that KCTD13 may regulate AR degradation or may act at the post-transcriptional level. KCTD13 KD in LNCaP cells showed decrease in the mRNA levels of two AR-dependent genes (NDRG1 and SGK1), both involved in cellular stress response, suggesting that loss of KCTD13 downregulates AR downstream activity. While cytoplasmic AR levels are increased with KCTD13 KD, western blot analysis of nuclear extracts of AR-HeLA cells show that nuclear AR levels are actually decreased at 20 and 60 mins after R1881 exposure suggesting that KCTD13 is inhibits AR translocation to the nucleus, thereby inhibiting its activity. Immunohistochemistry of AR-Hela cells confirm increased cytoplasmic and decreased nuclear AR levels in cells treated with R1881 at 30 and 60 minutes after R1881 exposure. F-actin staining of all four cell lines with KCTD13 knockdown shows abnormal speculated actin structure, suggesting that KCTD13 regulates cellular cytoskeleton, which is essential for AR translocation. Preliminary phenotypic analysis of Kctd13 null mice show significantly increased frequency of cryptorchidism compared to controls.
Conclusion: KCTD13 gene-dosage changes are found in a subset of children with GU anomalies. KCTD13 mediates AR signaling. Phenotypic analysis of kctd13 null mouse reveals increased frequency of cryptorchidism. 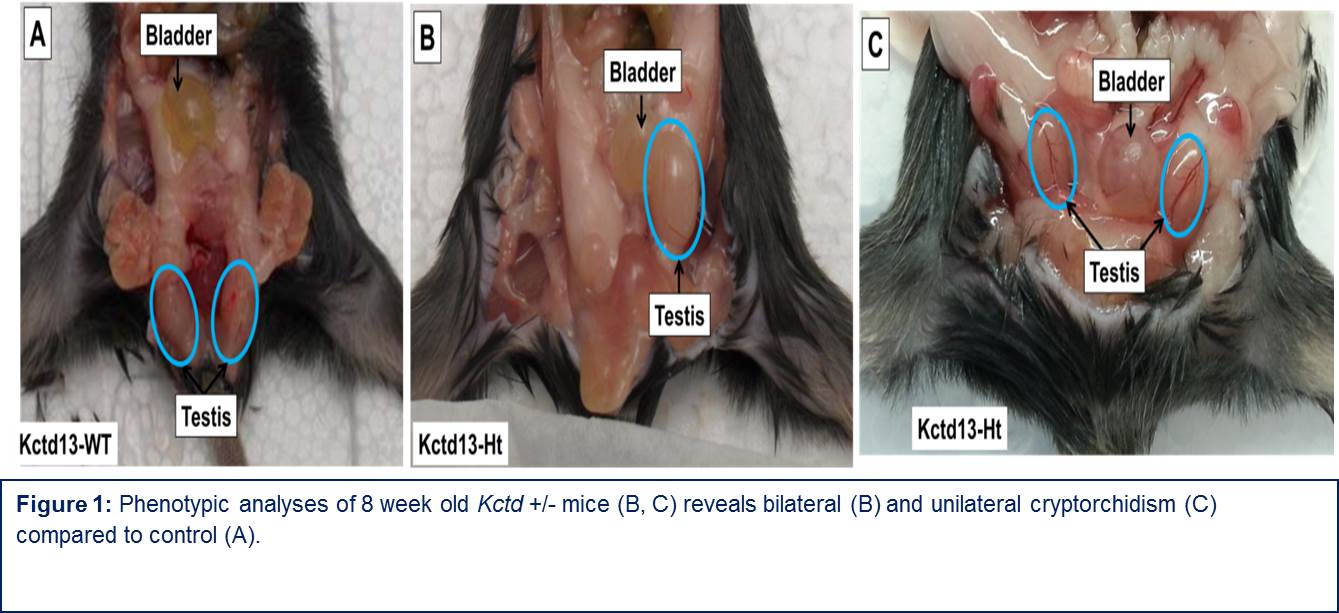
Back to 2016 Fall Congress
