Lack of Tcf21 Disrupts Bladder Organogenesis
Elizabeth Mann, PhD, Melissa Mogle, B.S., Joo-Seop Park, PhD, Pramod P. Reddy, M.B.B.S..
Cincinnati Children's Hospital Medical Center, Cincinnati, OH, USA.
BACKGROUND: Formation of the bladder depends on a continuum of reciprocal interactions between the epithelial and mesenchymal cell layers. Signaling molecules secreted from the urothelium are crucial for ongoing processes of both growth and differentiation of mesenchymal cells, including the patterning of detrusor smooth muscle cells. Less is known concerning the role of mesenchymal factors in this process. We describe here the role of the mesenchymal transcription factor Tcf21 in bladder development.
METHODS: We used a mouse model in which the Tcf21 gene is disrupted by a LacZ insertion (Quaggin et al., Development 126, 5771-5783, 1999). We studied bladder development using embryonic mice from timed heterozygote matings. We examined bladder size, the appearance of urothelial and muscle markers by immunofluorescence (IF) and quantitative PCR, and proliferation using Ki67 immunostaining. Statistical significance was measured by t-test analysis.
RESULTS: Tcf21 expression in the developing bladder, marked by surrogate LacZ staining, is confined to the mesenchyme. At E14.5, robust expression of the smooth muscle markers α smooth muscle actin (SMA) and transgelin, and the urothelial markers uroplakin 2 (UPK2) and Trp63, is seen by IF in control bladders. In contrast, while urothelial markers appear appropriately expressed, there is little to no expression of smooth muscle markers in the Tcf21 null bladder. Quantitative PCR (Figure 1) revealed decreased expression of muscle marker genes SMA, smooth muscle myosin, and calponin compared to control while there was no change in the level of transgelin transcripts. Of note, there was decreased expression of several members of the uroplakin gene family. At E16.5, robust expression of SMA and UPK 2 was present by IF in both genotypes. Bladder size is reduced in Tcf21 null mice. While a decrease of about 30% in cell number was seen at E14.5, there was no difference in the percent of Ki67-positive nuclei, suggesting that proliferation is undisturbed.
CONCLUSIONS: At E14.5, the differentiation of smooth muscle cells is delayed in the Tcf21 null bladder. There is a corresponding decrease in the transcript level of several muscle specific genes. While differentiation recovers by E16.5, we have shown that Tcf21 is important for the correct timing of smooth muscle differentiation in the bladder, and it may play an early role in mesenchymal/epithelial cell patterning. In addition, Tcf21 disruption leads to decreased total bladder size which does not correlate with the unchanged proliferation seen at E14.5. Further study of the Tcf21 null mouse model will help to elucidate molecular pathways crucial for normal bladder growth and detrusor muscle formation.
Figure 1. Expression of both smooth muscle genes and urothelial genes in the bladder at E14.5 is decreased by lack of Tcf21. Acta2 (α smooth muscle actin), Myh11 (smooth muscle myosin), Cnn1 (calponin), Tagln (transgelin), Myocd (myocardin), Upk (uroplakin), Foxa1 (forkhead box A1). 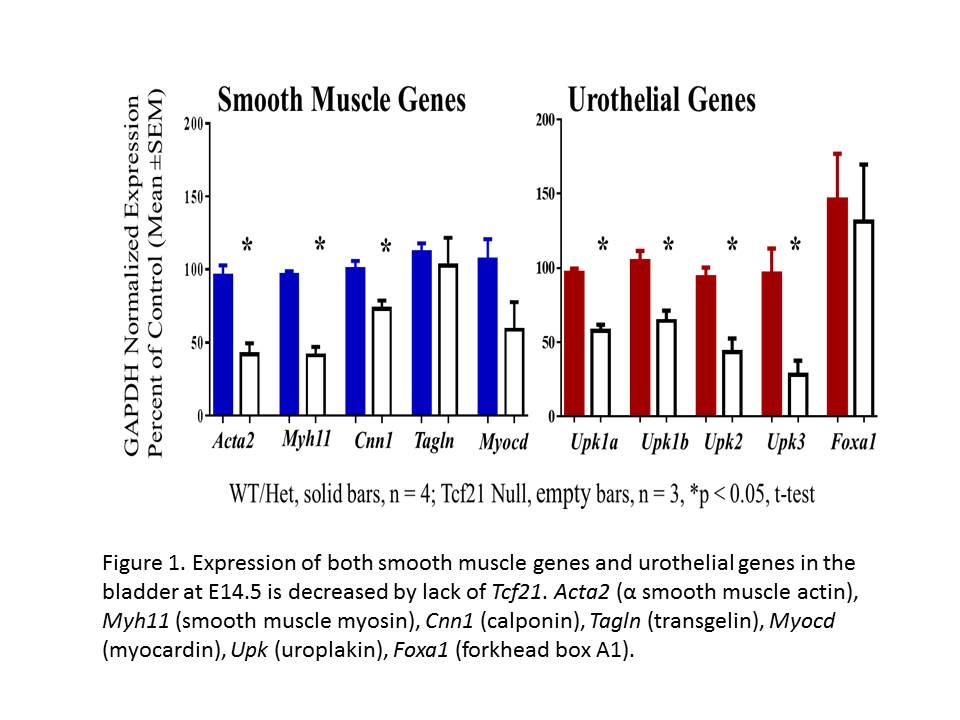
Back to 2016 Fall Congress
