Cost Comparison of Intra-Detrusor Injection of Botulinum Toxin Versus Augmentation Cystoplasty for Refractory Neurogenic Detrusor Overactivity
Adam J. Kern, M.D.1, Kunj Sheth, M.D.1, Micah A. Jacobs, M.D., M.P.H.1, Ardavan Akhavan, M.D.2.
1University of Texas Southwestern Medical Center, Dallas, TX, USA, 2Johns Hopkins Medical Institutes, Baltimore, MD, USA.
Background: Treatment options for refractory detrusor overactivity (DO) among children with neurogenic bladder include botulinum toxin type A (BTX) and augmentation cystoplasty (AC). Although BTX is accepted in contemporary pediatric urologic practice, cost-comparison and long-term outcome data for BTX is limited relative to the gold standard. The purpose of this study was to model and compare anticipated costs of AC vs. BTX over time, using published cost and effectiveness data, to compare the durability of BTX with AC.
Methods: The base assumption is a pediatric patient with refractory neurogenic DO. Input variables included outcome probabilities and cost ranges derived from published sources. A decision-analysis model was generated using TreeAge Pro Healthcare 2015 Software. The model was then rolled back to analyze relative cost, and sensitivity analysis was performed on the duration of therapy variable for BTX to ascertain the conditions of cost equivalence.
Results: Assuming 1 BTX treatment every 6 months, the average cost for 1 year of BTX with no treatment complication is $4,194.80. BTX treatment complications add a cost average of $329.29. The cost of BTX failure requiring further therapy was also modeled, with a yearly cost of daily anticholinergic of $2,227.00 and a net AC cost $100,713.93. The lifetime cost of AC was derived to be $72,903.00, rising to $108,835.96 when accounting for anticipated complications. Cost equivalence was achieved between BTX and AC after 20 years of BTX, assuming no AC complications (BTX=$74,753.46), and after 28 years when AC complications are factored (BTX=$99,297.75).
Conclusions: Although there is significant heterogeneity of costs and charges between practice settings, modeling suggests that, if clinically effective for an individual pediatric patient with neurogenic DO, BTX does not achieve cost equivalence with AC until that child is well into adulthood, suggesting the cost-effectiveness of BTX in well-selected pediatric patients over time.
Figure 1—Influence Diagram for Cost Analysis Model
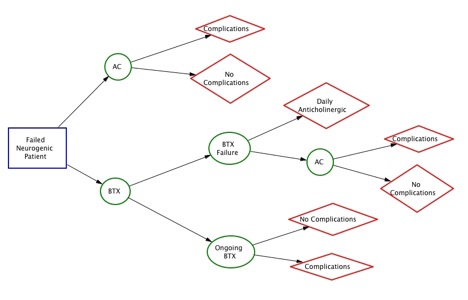
Figure 2—Estimates of Cost and Probabilities Used In Model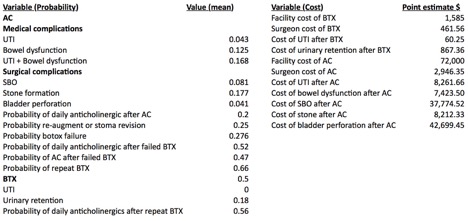
Back to 2016 Fall Congress
