Optogenetic Stimulation of Corticotropin-Releasing Hormone Expressing Neurons in Barrington's Nucleus Recapitulates the Social Stress Voiding Phenotype in Mice
Jason P. Van Batavia, MD, Stephan Butler, MS, Eleanor Lewis, BA, Joanna Fesi, BA, Rita Valentino, PhD, Stephen A. Zderic, MD.
The Children's Hospital of Philadelphia, Philadelphia, PA, USA.
BACKGROUND: Lower urinary tract dysfunction (LUTD) affect 20% of "normal" school-aged children. A commonly diagnosed LUT condition is voiding postponement in which children void infrequently with large volumes. This condition is modeled in mice subjected to social stress who show decreased voiding frequency and increased voided volumes along with increases in corticotropin-releasing hormone (CRH) expression in Barrington's nucleus (BN) (ie, the pontine micturition center). Many BN neurons co-localize CRH and glutamate and a recent optogenetic study provided evidence for a role of these specific BN neurons in micturition (Hou et al, Cell 2016; 167:73). Optogenetics is a technique to selectively stimulate cells or neurons of interest via light activated channel receptors (ie, channel-2 rhodopsin [Chr2]). Here we examined the effects of optogenetic manipulation of CRH BN neurons on the in vivo voiding phenotype and urodynamics in awake mice. We hypothesized that stimulating these neurons at higher frequencies (10-50Hz) would lead to CRH-dependent alterations in voiding phenotype (ie, larger voided volumes and longer intermicturition intervals.
METHODS: Double transgenic mice expressing channel-2 rhodopsin (Chr2) in CRH cells were generated using the Cre-lox recombinase system and had fiberoptic probes implanted into BN at 8 weeks of age. The mice also underwent simultaneous catheter placement into the bladder for in vivo cystometry. In vivo cystometry before and during optogenetic stimulation at various frequencies was performed 5-7 days postoperatively. Saline was perfused at 10Ál/min and baseline stable voiding cycles were established. Bladder capacity, threshold pressure, voiding pressure, and voided volume were recorded at baseline and at each optogenetic setting. In some mice, the protocol was repeated in the presence of CRH antagonist (NBI 30775).
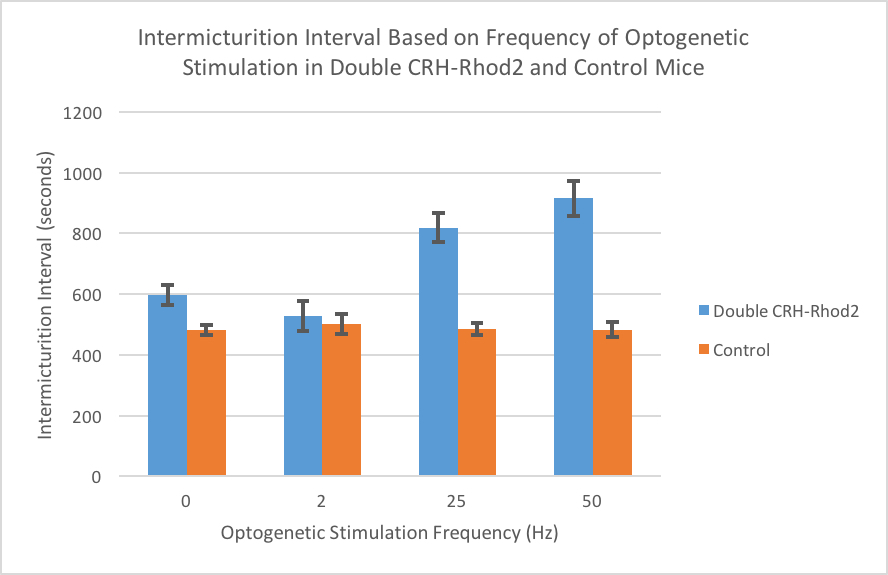
RESULTS: Fiberoptic stimulation (470 nm at 25 and 50 Hz) produced a significant rise in the intermicturition interval, bladder capacities and increased void volumes (Figure 1 and Table 1). This effect was especially pronounced in females in whom bladder capacity and intermicturition interval more than doubled at 50Hz stimulation. Fluoroscopic images confirmed complete bladder emptying with each void. The increased bladder capacity at higher frequencies (25 and 50Hz) was CRH-dependent as injection of a CRH antagonist (NBI 30775) blocked the optogenetic effect. Control non-double mice showed no effects from optogenetic stimulation.
CONCLUSIONS: Our results suggest that optogenetic stimulation of CRH BN neurons at higher frequency (25 and 50Hz) stimulations inhibits micturition and recapitulates the voiding phenotype seen in socially stressed mice (large, infrequent voids) with no effects noted at 2 Hz. Further elucidation of the neurons in BN are warranted to understand micturition and how it may be manipulated in disease states such as infrequent voiding and acute urinary retention.
Back to 2017 Program
