Tlr4 Dependent IL-6 Signaling Impacts Uropathogen Fitness Through Inhibition of Intracellular Bacterial Invasion
Christina Ching, MD, Sudipti Gupta, PhD, Rollin Li, MS, Brian Becknell, MD PhD.
Nationwide Children's Hospital, Columbus, OH, USA.
BACKGROUND: Innate immunity serves a critical role during UTI pathogenesis. Detection of uropathogenic bacteria by pattern recognition receptors, such as Toll-like receptor 4 (Tlr4), engages intracellular signaling pathways important in orchestrating the immune response. Urinary and systemic levels of interleukin-6 (IL-6) increase during human and experimental UTI with the level of IL-6 induction inversely proportional to bacterial virulence. This suggests uropathogen fitness depends on the ability to suppress IL-6 production; however, the function of IL-6 during UTI remains unclear. We hypothesized that IL-6 affects bacteria invasion and that IL-6 signaling would be dependent on Tlr4 signaling. We thus evaluated the impact of IL-6 in urothelial cell bacterial invasion using in vivo and in vitro models of uropathogenic E. Coli (UPEC) UTI and evaluated IL-6 signaling in a Tlr4 hyporesponsive system. METHODS: We infected 6 week old female wild type (WT) C57BL/6J or IL-6 knock out (KO) mice transurethrally with UPEC and sacrificed at variable time points. IBC enumeration was performed using β-galactosidase staining to determine the dependence of IBC formation on IL-6. We then tried to reverse the phenotype in IL-6 KO mice with intravesical recombinant IL-6 (vs. PBS) given 1 hour before and 2 hours post UPEC inoculation (hpi). Bladders were harvested 6 hpi. We confirmed our findings in vitro using 5637 urothelial cells. We performed a bacterial invasion assay in cells stimulated with either IL-6 or PBS 30-minutes prior to UPEC inoculation. Cells underwent 1 hour invasion phase followed by 1.5 hour gentamicin incubation. Cells were then lysed and analyzed by dilution plating to determine intracellular bacterial burden. Finally, the requirement for Lipopolysaccharide (Lps) / Tlr4 signaling in IL-6 induction and downstream target Stat3 phosphorylation was established using Lps-hyporesponsive C3H/HeJ mice and C3H/HeOuJ Lps-sensitive controls and evaluated by ELISA, Western blotting, immunohistochemistry, and bacterial burden. Results were evaluated by Mann-Whitney U test with p <0.05 being significant.
RESULTS: There was an initial (6 and 16 hpi) significant difference in IBC enumeration between IL-6 KO and WT mice that normalized at 24hpi (Figure 1). Fewer IBCs formed in IL-6 KO mice with intravesical IL-6 as compared to PBS. Exogenous IL-6 stimulation given in vitro inhibited bacterial urothelial invasion with less bacterial burden recovered in IL-6 compared to PBS stimulated cells. We confirmed that Tlr4 signaling is necessary for IL-6 induction/Stat3 phosphorylation in C3H/HeOuJ mice as compared to HeJ mice, with a significant difference in bacterial burden between mouse strains. Bladder bacterial burden in HeJ mice was reduced with recombinant IL-6.
CONCLUSIONS: IL-6 directly interferes with UPEC urothelial invasion and IBC formation.Tlr4 signaling is necessary for IL-6/Stat3 expression. Elevated acquisition of IBC and bacterial burden in Tlr4 hyporesponsive models is a result of alterations in the IL-6 signaling pathway which can be rescued with restoration of exogenous IL-6. We implicate that variations in IL-6 signaling could explain UTI susceptibility.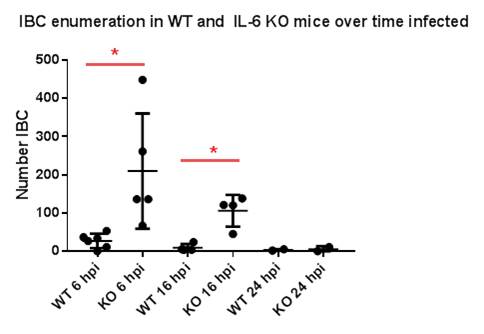
Back to 2017 Program
