IPSE, a Urogenital Parasite-Derived Protein, Drives Urothelial Proliferation and Alleviates Chemotherapy-Induced Hemorrhagic Cystitis in a Nuclear Localization Sequence-Dependent Fashion, and Anesthetizes Against Somatic Inflammatory Pain
Evaristus C. Mbanefo, PhD1, Loc Le, PhD1, Nirad Banskota, MS1, Luke F. Pennington, BS2, Abdulaziz Alouffi, BS3, Debalina Ray, PhD4, David Meery, PhD3, Theodore Jardetzky, PhD2, Franco Falcone, PhD3, Michael Hsieh, MD/PhD5.
1Biomedical Research Institute, Rockville, MD, USA, 2Stanford University, Stanford, CA, USA, 3University of Nottingham, Nottingham, United Kingdom, 4BioVision, Milpitas, CA, USA, 5Children's National Medical Center, Washington, DC, USA.
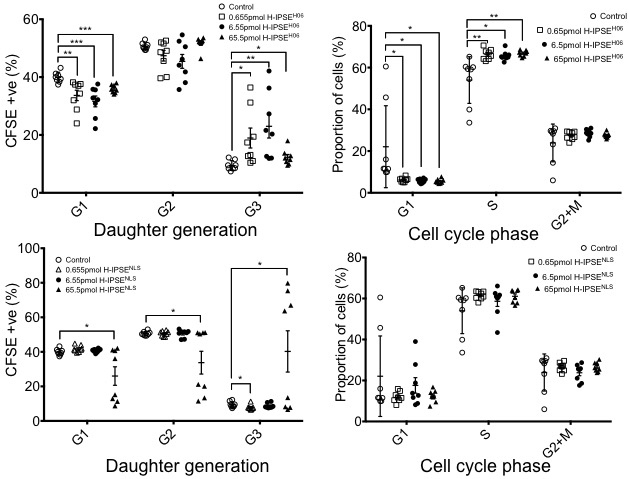
Background: Inflammatory pain, including chemotherapy-induced hemorrhagic cystitis, is a feature of many urologic diseases affecting children. Mesna-refractory hemorrhagic cystitis is often difficult to manage, and thus there is a need for alternatives to Mesna. We previously reported that a single dose of H-IPSEH06, hereafter called wild type H-IPSE (wt H-IPSE), a Schistosoma haematobium-derived host immunomodulatory protein, is superior to three doses of Mesna in preventing ifosfamide-induced hemorrhagic cystitis. Herein we expand upon this work in three new directions: 1) characterization of wt H-IPSE's influence over urothelial proliferation; 2) delineation of unique gene expression patterns associated with nuclear localization sequence(NLS)-dependent H-IPSE effects on the ifosfamide-injured bladder; and 3) demonstration that a single wt H-IPSE dose decreases somatic inflammatory pain. Methods: Recombinant wt H-IPSE or an NLS mutant of IPSE (mutant H-IPSE) was incubated with mouse and human urothelial cell lines, and proliferation and cell cycle status measured by flow cytometry using CFSE and propidium iodide, respectively. Mice were administered wt or mutant H-IPSE, challenged with ifosfamide, sacrificed, and their bladder RNA isolated and subjected to RNA-Seq analysis. Other mice were given wt H-IPSE or vehicle followed by footpad injections of carrageenan (to induce inflammatory pain) or vehicle, and then tested for pain responses in a blinded fashion. Results: wt H-IPSE increased both mouse and human urothelial cell proliferation, and drove cells towards S phase (Figure 1). These effects were NLS dependent, and are consistent with wt H-IPSE's ability to protect the urothelium following ifosfamide challenge. RNA-Seq analyses revealed that several gene members of the MAPK pathway were differentially expressed between wt H-IPSE- vs. vehicle-treated mice challenged with ifosfamide. Analysis of RNA-Seq data also indicated that multiple muscle contraction-related gene pathways are differentially expressed between wt H-IPSE- vs. mutant H-IPSE-treated mice challenged with ifosfamide (Table 1). These findings are consistent with wt H-IPSE's ability to prevent ifosfamide-induced bladder dysfunction. Finally, a single injection of wt H-IPSE suppressed carrageenan footpad injection-induced acute and chronic pain (Figure 2 and Table 2).
Conclusions: wt H-IPSE continues to prove to be a promising prophylactic against chemotherapy-induced hemorrhagic cystitis. Our mechanistic studies on wt H-IPSE suggest potential means by which to optimize this molecule's NLS-dependent therapeutic effects. The ability of wt H-IPSE to alleviate somatic inflammatory pain expands its therapeutic potential to include use as a non-opioid analgesic, an especially exciting application given the ongoing opioid crisis.
| Gene pathway | Gene Count | % Genes Differentially Expressed | P-value | Benjamini |
| Arrhythmogenic right ventricular cardiomyopathy | 6 | 4.2 | 8.8E-5 | 8.9E-3 |
| Hypertrophic cardiomyopathy | 5 | 3.5 | 1.6E-3 | 8.0E-2 |
| Dilated cardiomyopathy | 5 | 3.5 | 2.0E-3 | 6.5E-2 |
| Hippo signaling pathway | 6 | 4.2 | 2.8E-3 | 6.9E-2 |
| Regulation of actin cytoskeleton | 6 | 4.2 | 1.2E-2 | 2.2E-1 |
| Proteoglycans in cancer | 5 | 3.5 | 4.2E-2 | 5.2E-1 |
| Vascular smooth muscle contraction | 4 | 2.8 | 4.9E-2 | 5.2E-1 |
| Cardiac muscle contraction | 3 | 2.1 | 8.9E-2 | 6.9E-1 |
| cGMP-PKG signaling pathway | 4 | 2.8 | 9.8E-2 | 6.9E-1 |
| Comparison | Baseline | Day 1 post-carrageenan injection | Day 3 post-carrageenan injection | Day 6 post-carrageenan injection | Day 22 post-carrageenan injection |
| Control vs. vehicle+carrageenan | NS | p<0.0001 | p=0.0003 | p=0.0026 | p=0.013 |
| Control vs. wt H-IPSE + carrageenan | NS | p=0.0011 | p=0.0186 | NS | NS |
| Vehicle+carrageenan vs. wt H-IPSE+ carrageenan | NS | p<0.0001 | p<0.0001 | p=0.0047 | p=0.0048 |

Back to 2017 Program
