Bladder Stem Cells Are Regulated By Androgens In A Murine Model Of Bladder Outlet Obstruction
Paula R. Firmiss, BS1, Diana K. Bowen, MD2, Natalie Kukulka, BA1, Robert W. Dettman, PhD2, Edward M. Gong, MD3.
1Lurie Children's Hospital, Chicago, IL, USA, 2Northwestern University, Chicago, IL, USA, 3Northwestern University & Lurie Children's Hospital, Chicago, IL, USA.
Introduction
Boys with posterior urethral valves often develop bladder deterioration requiring clean intermittent catheterization following the onset of puberty. Previously our lab has demonstrated that bladder muscle fibrosis in the setting of partial outlet obstruction (PO) is testosterone dependent. Furthermore, we have identified a progenitor mesenchymal stem cell population in murine bladders (bladder stem cell, BSC) that respond to partial bladder outlet obstruction (PO). In this study, we investigate the role of androgens upon these BSCs in the setting of bladder outlet obstruction.
Methods
8-10 week male CD-1 mice were surgically obstructed at the bladder neck to create a partial obstruction model (PO), with and without surgical castration (CPO). Success of PO was determined after 1 week by voiding stain on paper analysis. Testosterone cypionate was administered to a CPO group at time of castration (CPOT). Fluorescence-activated cell sorting (FACS) was utilized to isolated BSCs (Sca-1+/ CD34+/ lin- (PECAM-, CD45-, Ter119-)) in the sham, PO, CPO, and CPOT bladders after 7 days of obstruction. BSCs were cultured for 24 hours and expression of androgen receptor (AR) on the BSCs was determined with immunofluorescence. Quantitative PCR was performed to determine levels of gene expression.
Results
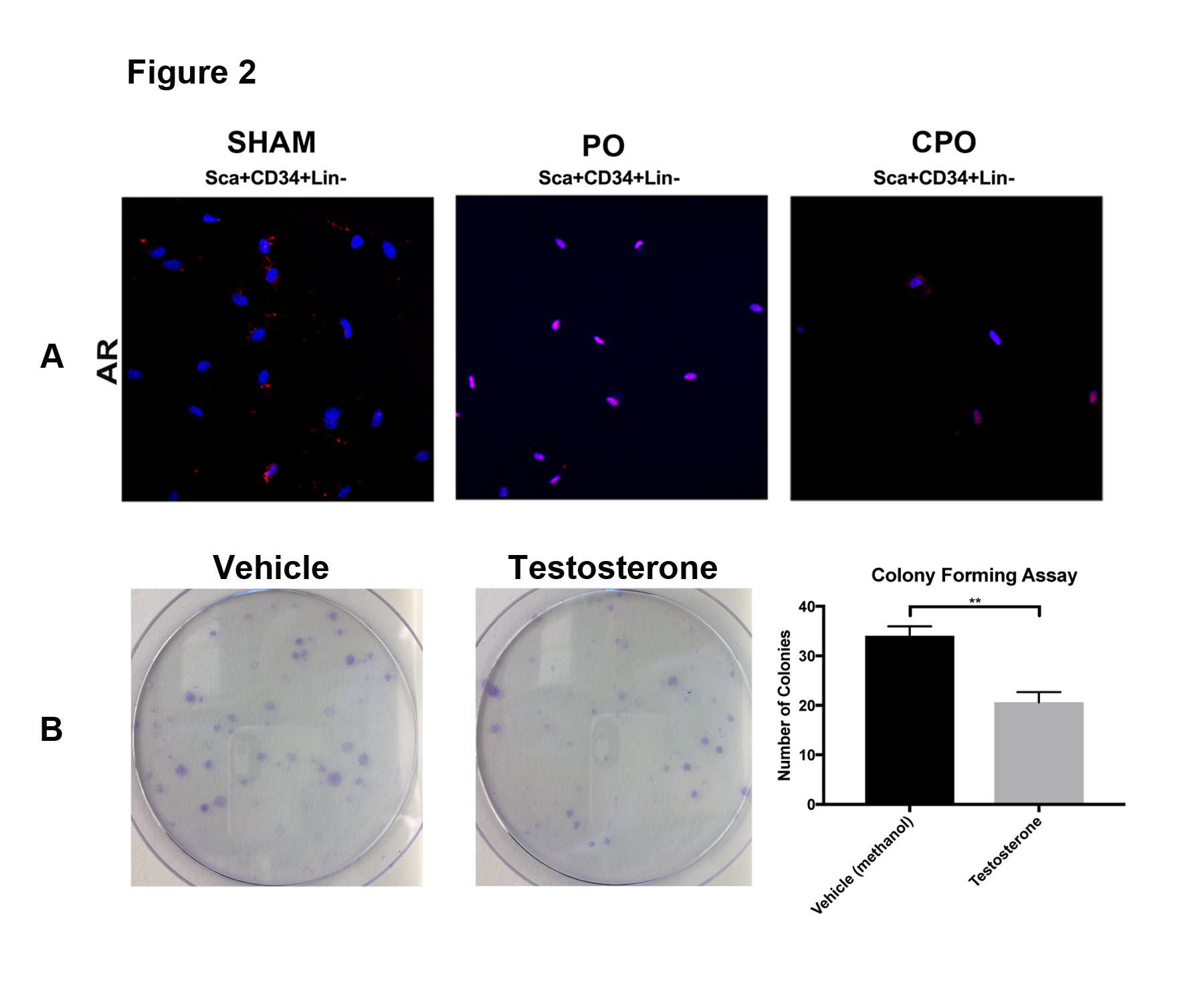
Obstruction decreased the BSC population and increased fibrosis relative to sham animals. These effects were reversed in obstructed, castrated mice (CPO) where the BSC population was maintained and fibrosis was decreased. Restoration of testosterone in CPO mice led to a concomitant decrease in the BSC population (Figure 1A, B, C). Twice as many BSCs expressed AR (80%) after obstruction when compared to sham (40%) (Figure 2A). In the presence of testosterone, BSCs were hindered in their ability to form colonies (Figure 2B).
Conclusions
Our data suggest that testosterone plays a direct role in the regulation of BSCs in the context of bladder outlet obstruction. This conclusion is supported by the decrease in BSCs and bladder fibrosis following obstruction, restoration of BSCs following castration, and loss of BSCs when testosterone is supplemented. These findings could provide new information regarding maladaptive bladder remodeling associated with obstruction. It may identify novel therapeutic targets to prevent bladder deterioration.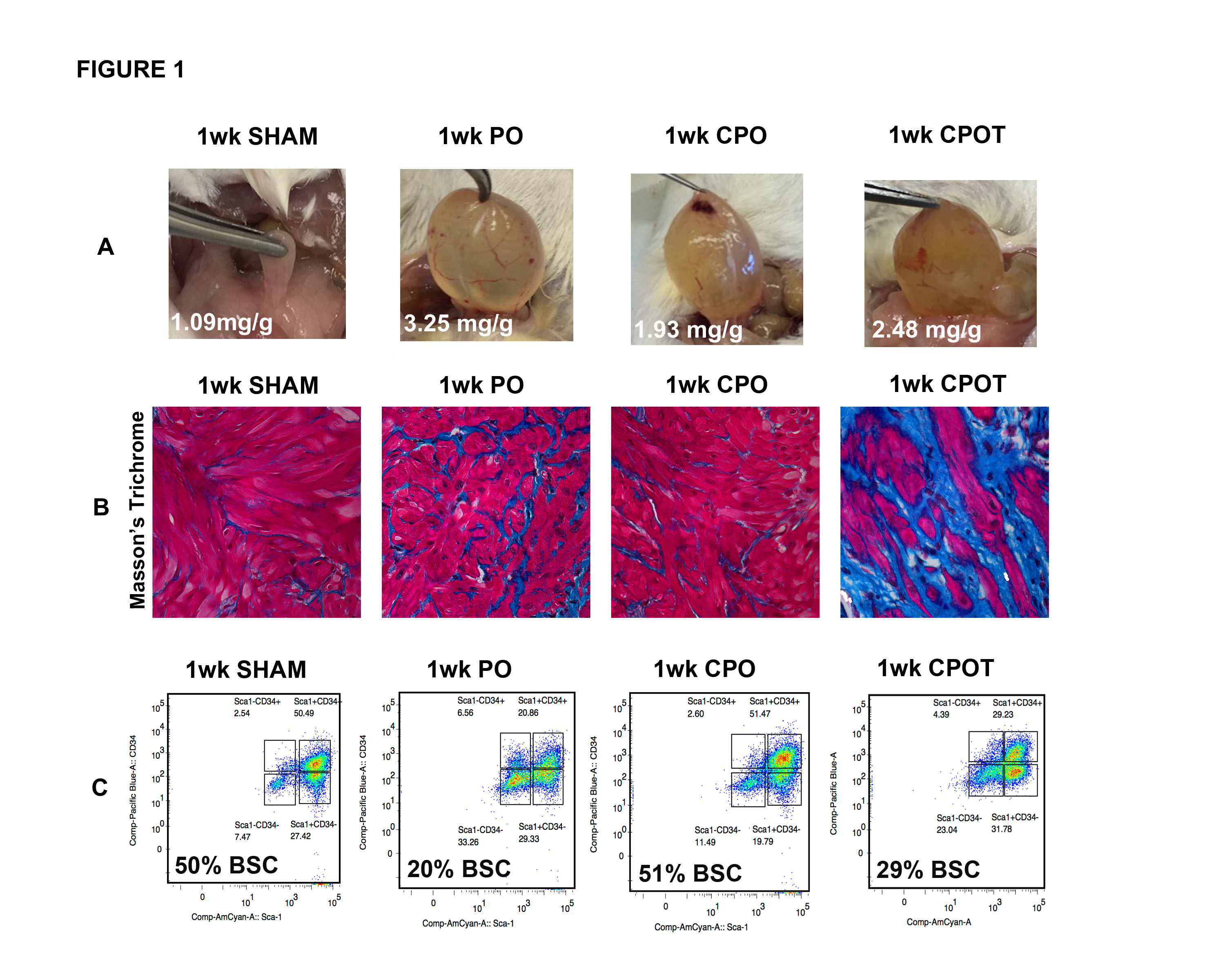
Back to 2017 Program
