Profiling Of Renal Stem Cell Markers In Mid-Gestational Human Fetal Kidney Using Clarity-Optimized Light-Sheet Microscopy (Colm)
Yan Liu, Ph.D., Xue-Ru Wu, M.D., Hongying Huang, M.D., Ellen Shapiro, M.D..
New York University School of Medicine, New York, NY, USA.
BACKGROUND: One of the goals of renal regenerative medicine is to identify and isolate human fetal nephron stem/progenitor cells which have the potential to regenerate and replenish epithelial cell types within the nephron. In the human fetal kidney (HFK), new nephrons are induced by the interaction between mesenchymal progenitor cells and collecting duct tips located in the periphery of the kidney. This leading edge of active nephron induction is known as the "nephrogenic zone" which consists of the collecting duct tips and the cap mesenchyme cells, a subpopulation of MM cells representing the pool of multipotent renal stem cells, with the ability to self-renew and differentiate into various types of nephron epithelia. CLARITY is a technique that allows molecular and structural interrogation of tissue by allowing deep imaging of biochemically labeled transparent intact tissue samples. Our objective of this study was to use CLARITY for the first time to identify renal progenitor populations in nephron cellular components of HFK using stem cell markers (NCAM1, SALL1, PAX2, CD133 and CD24) to elucidate their role in human nephrogenesis. MATERIALS AND METHODS: Human male fetal kidneys, 17 weeks gestational age, were perfused in hydrogel, embedded in polymerized hydrogel and passively clarified by clearing buffer. Clarified tissues were incubated with antibodies including anti- NCAM1, SALL1, PAX2, CD133, CD24, podocalyxin and anti-AQP1 and imaged by COLM. Data processing was performed by Zeiss Lightsheet Z.1 with ZEN software. Kidney volumetric reconstructions, renal tubule tracing and segmentation were performed using Imaris 3D Image Analysis Software and NIH ImageJ. RESULTS: COLM imaging of NCAM1 demonstrated predominant expression in the MM and renal stroma. In the nephrogenic zone, we observed strong expression in the MM and its derivatives including cap mesenchyme, renal vesicles, early S and comma shaped nephron figures and newly forming tubules that expressed proximal convoluted tubule marker, AQP1. SALL1 was localized to the nephrogenic zone including MM and its derivatives, and also to the differentiating nephrons located deeper within the kidney. PAX2 demonstrated predominant expression in the MM and its derivatives and stroma. Both SALL1 and PAX 2 were detected in some newly formed AQP1 positive (+) proximal tubules. CD24 was expressed in MM and its derivatives, but not in the stroma. CD24 was also localized in developing glomeruli and tubules and co-localized with podocyte marker, podocalyxin. CD133 was sparsely distributed in MM, as expected at this stage of development and the majority of CD133+ cells co-localized with CD24. There was a CD133+ subpopulation that did not express CD24 and was only localized in the collecting ducts. CONCLUSIONS: This is the first study of human fetal kidney using COLM to 3-D image the expression of renal stem cell markers. Precise localization of these proteins in specific renal cell populations and subpopulations provides new insights into fetal nephrogenesis and its therapeutic implications.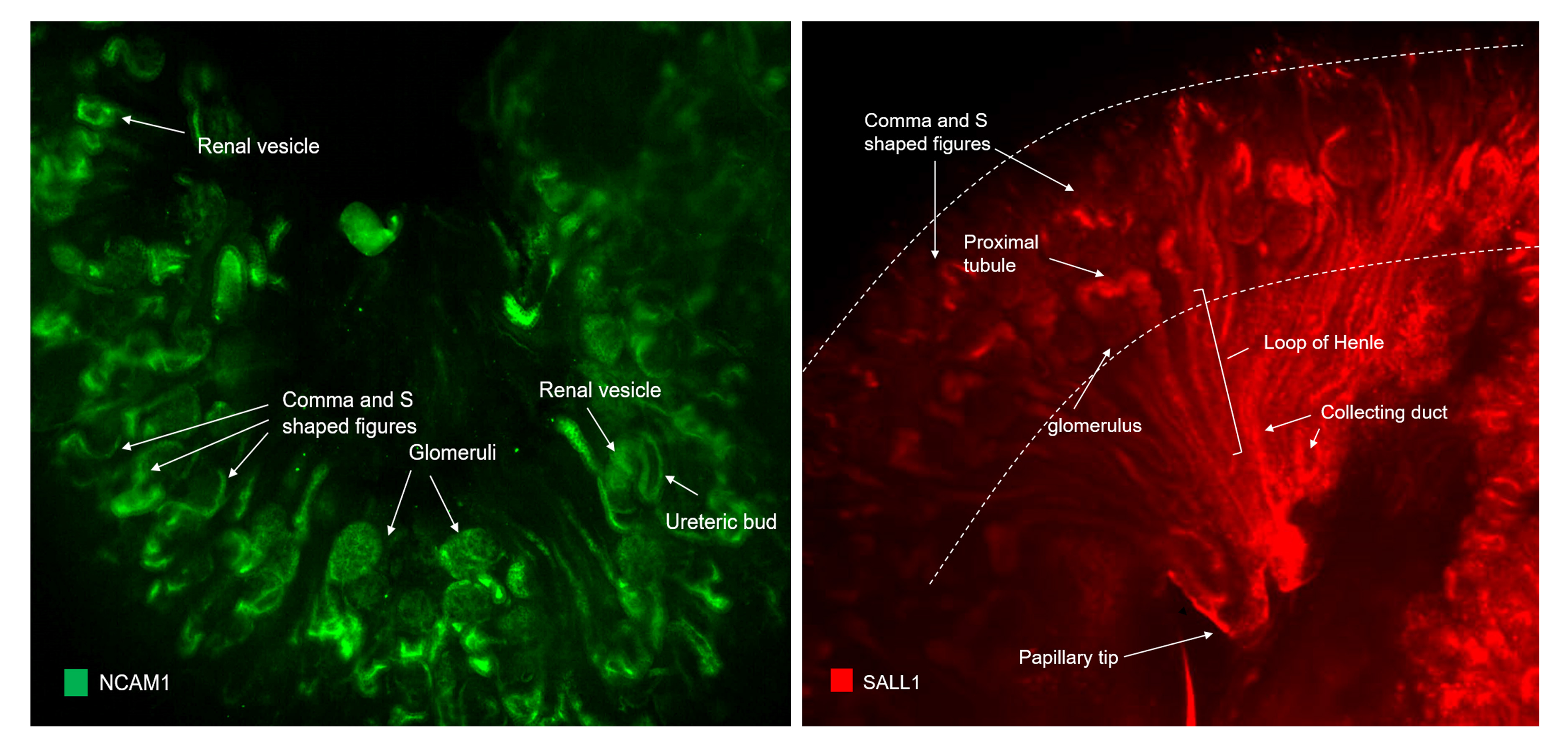
Back to 2017 Program
