-->
|
Back to 2014 Fall Congress Meeting Posters
Understanding the ramifications of postoperative ketorolac after pediatric pyeloplasty
Courtney K. Rowe, MD1, Jeffrey J. Leow, MD, MPH1, Constance S. Houck, MD2, Steven L. Chang, MD, MS1, Richard N. Yu, MD, PhD2.
1Brigham and Women's Hospital, Boston, MA, USA, 2Boston Children's Hospital, Boston, MA, USA.
BACKGROUND
The safety of ketorolac has been well documented for pediatric surgery, and its use has become commonplace for many urologic procedures including pyeloplasty. While complications are felt to be low, there are no contemporary population-based studies evaluating this issue.
We set out to examine the rate of complications after postoperative ketorolac use in a nationally representative sample of pediatric pyeloplasty. This included major complications as well as very minor deviations from standard care.
METHODS
Hospitalization data was examined from the Premier Database (Premier Inc, Charlotte, NC) for January 1, 2003 through December 31, 2012. The Premier network encompasses more than 2900 hospitals, representing approximately 20% of discharges from nonfederal institutions in the United States. Patients <= 18 years of age who underwent pyeloplasty (ICD9 55.87) were included.
The billing charge master for each patient was reviewed to identify medications and phlebotomy charges. We chose to record incidence of medication use as weight was not available. Complications within 90 days were included by examining longitudinal data on readmissions and emergency room visits. Survey weights were applied and hospital clustering adjusted for.
Patients who received ketorolac during their hospitalization formed the primary cohort. We excluded patients with a prior diagnosis of renal insufficiency (ICD9 581, 582, 583, 585, 586, 587, 589) and those who received other NSAIDs during their hospitalization.
RESULTS
There were a sample weighted estimate of 12,357 patients who met criteria, 36.3% of whom received ketorolac immediately post-operatively. The ketorolac group was older, with a mean age of 8.1 years compared to 4.5. Operative time was slightly higher in the ketorolac group, but this did not reach significance (215 vs. 206, p = 0.147). Length of stay was approximately 2.5 days for both groups. Most patients (92.3% vs 94%, p = 0.114) had no complications. Opioid usage was similar, with a mean 2 doses per day hospitalization. Slightly less than half of all patients had blood drawn during their hospitalization, and about half had some sort of radiologic study, primarily an X-ray or CT scan.
CONCLUSIONS
While we did not find any reduction in length of stay, cost or opioid use, we also did not find any increase in complications in the ketorolac cohort. There was no evidence of minor deviations from standard post-operative care, including no increase in lab draws or radiologic studies. Overall, ketorolac appears to be safe after pediatric pyeloplasty.
Table 1 - Patient and hospital characteristics
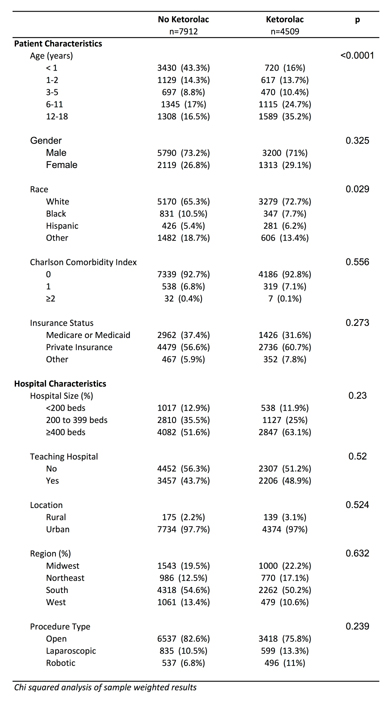
Table 2 - Postoperative outcomes
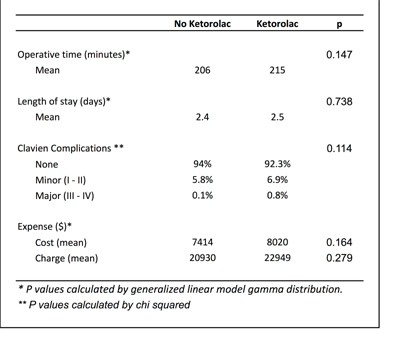
Table 3 - Deviations from standard care

Back to 2014 Fall Congress Meeting Posters
|




