Next-Generation Patient Avatars for Understanding Heterogeneities in Chemotherapy Responses in Wilms Tumor and Pediatric Rhabdomyosarcoma
Candace Granberg, M.D., Patricio C. Gargollo, M.D., Slavic Fedyshyn, Sr Res Tech, Matthew A. Lowerison, RS-Fellow, Mario A. Cepeda, RS-Fellow, Tijani Osumah, M.D., Mohamed Ahmed, RS-Fellow, Julie Byrne, CRC, John C. Cheville, M.D., Hon S. Leong, Ph.D..
Mayo Clinic, Rochester, MN, USA.
BACKGROUND: Cancer cell lines do not readily simulate the original disease due to passage of the cell line beyond the natural history of the cancer. We sought to understand and outmaneuver drug resistance by establishing a patient-derived xenograft platform for patients with Wilms Tumor (WT) and Pediatric Rhabdomyosarcoma (RMS). We propose the use of chick embryos (FigA) as opposed to immunocompromised mice, as hundreds of avatars can be generated per patient tumor due to high tumor take rates (>85%), low cost per avatar ($1/host), short timeframe needed to evaluate systemic therapies, and retainment of original tumor architecture. This permits studies of tumor heterogeneity, wherein various regions of the primary tumor or metastases can be individually treated with drug to determine if functional heterogeneity exists between various regions when challenged with drug (FigB).
METHODS: Primary tumors or metastases from WT (n=2) and RMS patients (n=2) were obtained via nephrectomy and/or metastasectomy. Various cores (1 cm diameter) were obtained from each primary tumor or metastasis. Each core was subdivided into 2-3mm fragments, then implanted into the chorioallantoic membrane (CAM) of Day 9 chick embryos, resulting in N=36 hosts/region of a tumor. In instances where cores could not be collected for various regions of a primary tumor, such as resected metastases, the entire specimen was subdivided into 2-3 mm tumor fragments and all fragments were implanted. Cell lines were generated from each tumor, and a commercially available RMS cell line, G401 was used to form xenografts as a control. Monotherapies (Vincristine, D-actinomycin, Cyclophosphamide, Doxorubicin, saline vehicle) were used to treat PDXs to achieve clinically relevant Cmax’s for each drug. Treatment cycles consisted of topical application of drug (4 µL) to the surface of the PDX once on Day 13 embryos and again on Day 15 embryos. At endpoint, Day 17 embryos were submitted to high-frequency ultrasound imaging to quantitate changes in tumor volume and tumor vascularity after treatment and compared to saline vehicle control xenografts.
RESULTS: Both PDXs and cell lines either formed by via primary culture of the primary/metastases or commercially available cell lines generated viable tumors within 1-2 days of implantation (FigC) into the CAM, based on high-frequency ultrasound imaging of tumor perfusion (FigD) and histology (FigE). Treatment with individual chemotherapy revealed heterogeneous responses throughout the PDXs with an overall decrease in tumor volume observed. PDXs yielded histological findings that were more representative of WT or RMS whereas cell lines were uniformly similar throughout the tumor.
CONCLUSIONS: The chick embryo model is an excellent alternative to mice as patient avatars for pediatric oncology patients with WT or RMS. Given the rarity of these tumors, the development of primary cell lines will enable further drug resistance studies. Treatment with a cocktail of chemotherapies will be pursued to predict which patients will maximally benefit versus patients that should be offered immunotherapy instead. 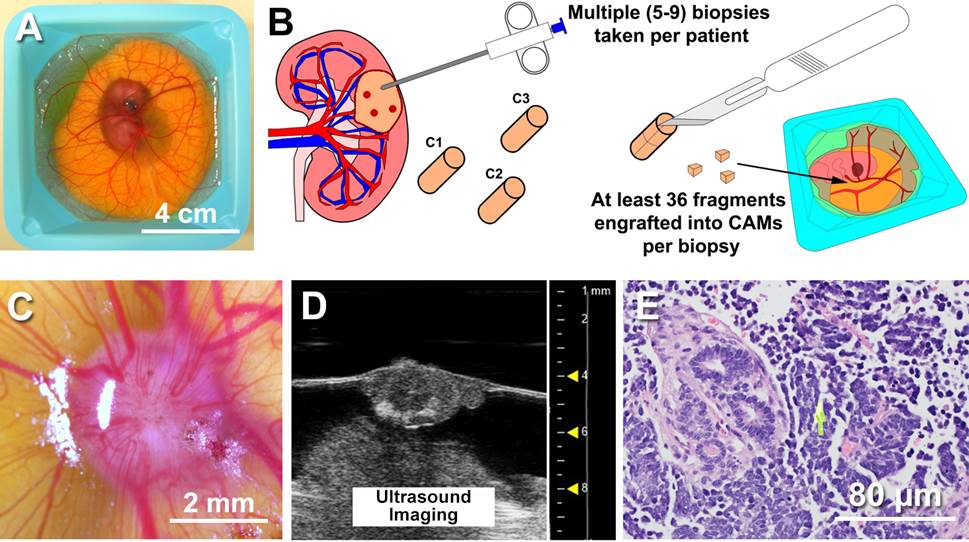
Back to 2018 Program




