A Simulated Model for Fluid and Tissue Heating During Pediatric Laser Lithotripsy
Jonathan Ellison, MD1, Brian MacConaghy, PhD2, Timothy Hall, PhD3, William Roberts, MD3, Adam Maxwell, PhD2.
1Seattle Children's Hospital, Seattle, WA, USA, 2University of Washington, Seattle, WA, USA, 3University of Michigan, Ann Arbor, MI, USA.
Background: Laser lithotripsy (LL) is a common modality for treatment of young children and adolescents with nephrolithiasis. Recent advances in laser technology now allow for higher power settings resulting in more efficacious “dusting” of renal and ureteral calculi. However, in vivo animal studies and simulations have shown rapid and sustained rise of fluid temperatures with LL, resulting in demonstrable evidence of tissue damage as seen histologically. The effect of LL on fluid and tissue heating in a pediatric model, however, is unknown. We hypothesize that smaller, pediatric kidneys will be more susceptible to changes in fluid temperature and therefore tissue damage, and that this effect will be age dependent.
Methods: Utilizing COMSOL Multiphysics finite-element modeling software, computational simulations were developed for LL in children, varying by child’s age (3, 8, and 12 years), flow of irrigation fluid (gravity - 5 mL/min or continuous pressure flow - 40 mL/min), and power settings (5W, 10W, 20W, 40W). This model uses a simplified axisymmetric geometry to represent the collecting space and has been previously validated against in vitro experiments. The model accounts for heat transfer via diffusion, convection, perfusion, and heat sourcing as well as tissue properties and blood flow of the urothelium and renal parenchyma. Laminar and heat-induced convection flow are simulated by the model, assuming room-temperature irrigation through the working channel of a ureteroscope. Renal size was varied by age, based on normative values as reported in the literature. Maximum fluid temperature after 60 seconds of simulated LL was captured. Thermal dose was calculated using the t43 equivalence of 240 minutes as a threshold for tissue damage, which is a common metric used for tissue viability in evaluation of hyperthermal or ablative effects. Based on this definition, tissue volume at risk for irreversible cellular damage was also estimated.
Results: Simulation revealed generation of thermal doses sufficient to cause tissue injury at gravity flow for all ages at 20W and 40W power settings. Higher temperatures were seen in younger ages across all power settings at gravity flow (see Figure). Within the renal pelvis, fluid heating with gravity flow resulted in potential for deep tissue injury at higher power across age groups, with volume of tissue injury ranging from 0.62 - 0.95 cm3 at 20W and 2.5 - 2.7 cm3 at 40W. Temperature increases were dampened with continuous pressure flow, with no thermal dose exceeding the threshold for injury. Correspondingly, minimal simulated tissue damage was produced under continuous pressure flow.
Conclusions: Smaller renal size is more susceptible to thermal changes induced by LL. However, power settings equal to or greater than 20 W can result in temperatures high enough for tissue damage at any age. Continuous pressure flow may provide dissipation of heat and thus mitigate the potential thermal damage from high power LL. 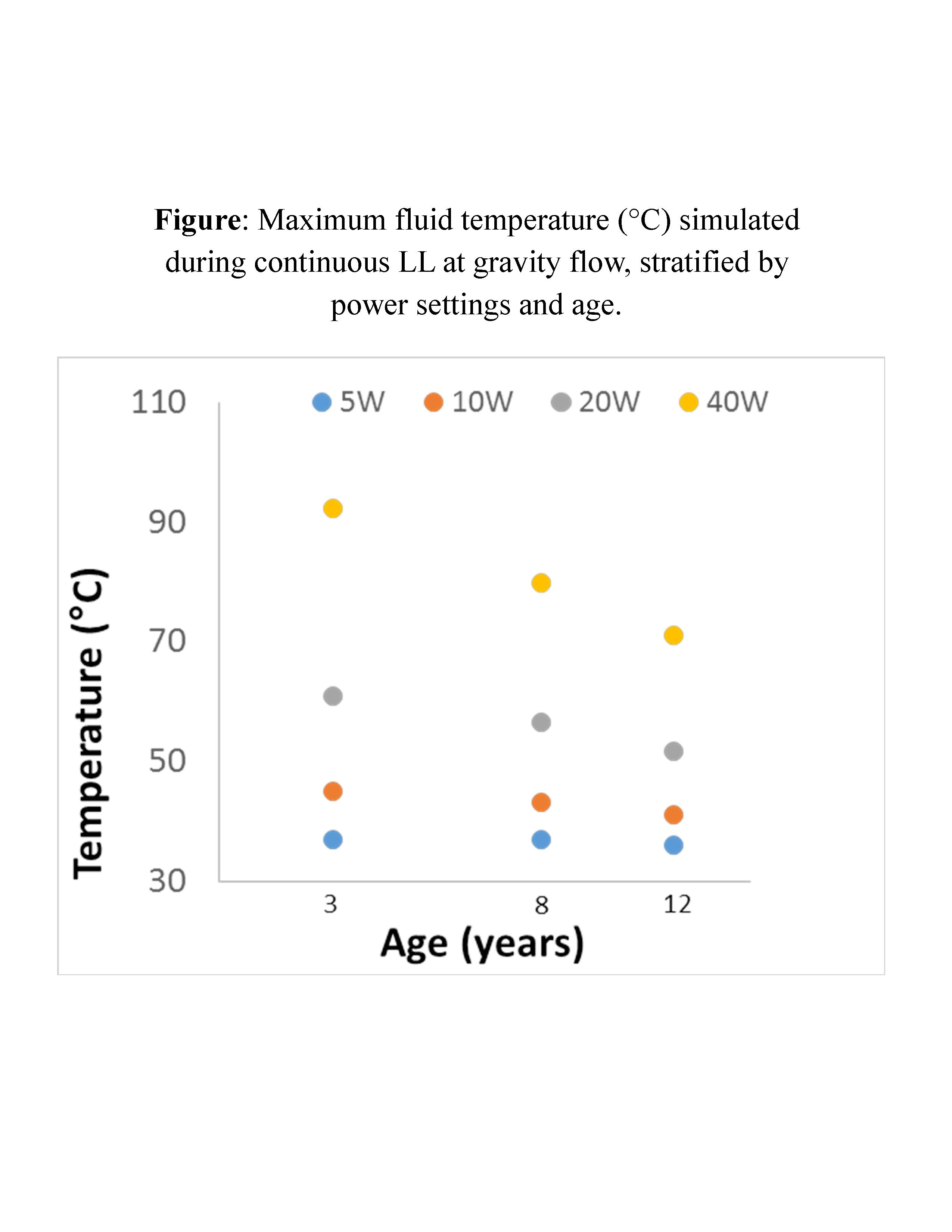
Back to 2018 Program




