Alterations of muscarinic and purinergic signaling in the bladder following early life bladder dysfunction
Nao Iguchi, PhD1, Duncan Wilcox, MD2.
1University of Colorado AMC, Aurora, CO, USA, 2Children's Hospital Colorado, Aurora, CO, USA.
BACKGROUND: Disturbance of bladder function in early life can contribute to lifelong urinary tract dysfunction and kidney failure. Although it is well recognized, the underlying mechanism was not well understood. Voiding in neonatal mice depends on the perigenital-bladder reflex triggered by their mother licking the perineum until voluntary bladder control emerges around 3-week-old. The objective of this study was to examine the effects of early life voiding perturbation including urinary retention and disturbing normal voiding cycle can be induced by neonatal maternal separation (NMS) using a murine model.
METHODS: Newborn mouse pups were divided into control and NMS groups after birth. NMS pups were removed from their dam and housed individually for 6 h per day from postnatal day 2 to 14 days. Pups in the control group stayed with their dam all the time. Long-term effects of NMS on bladder function were assessed in vivo by awake cystometry as well as in vitro by detrusor contractility studies at 6-week-old. Effects of NMS on gene expression in the bladder were also evaluated at 6-week-old.
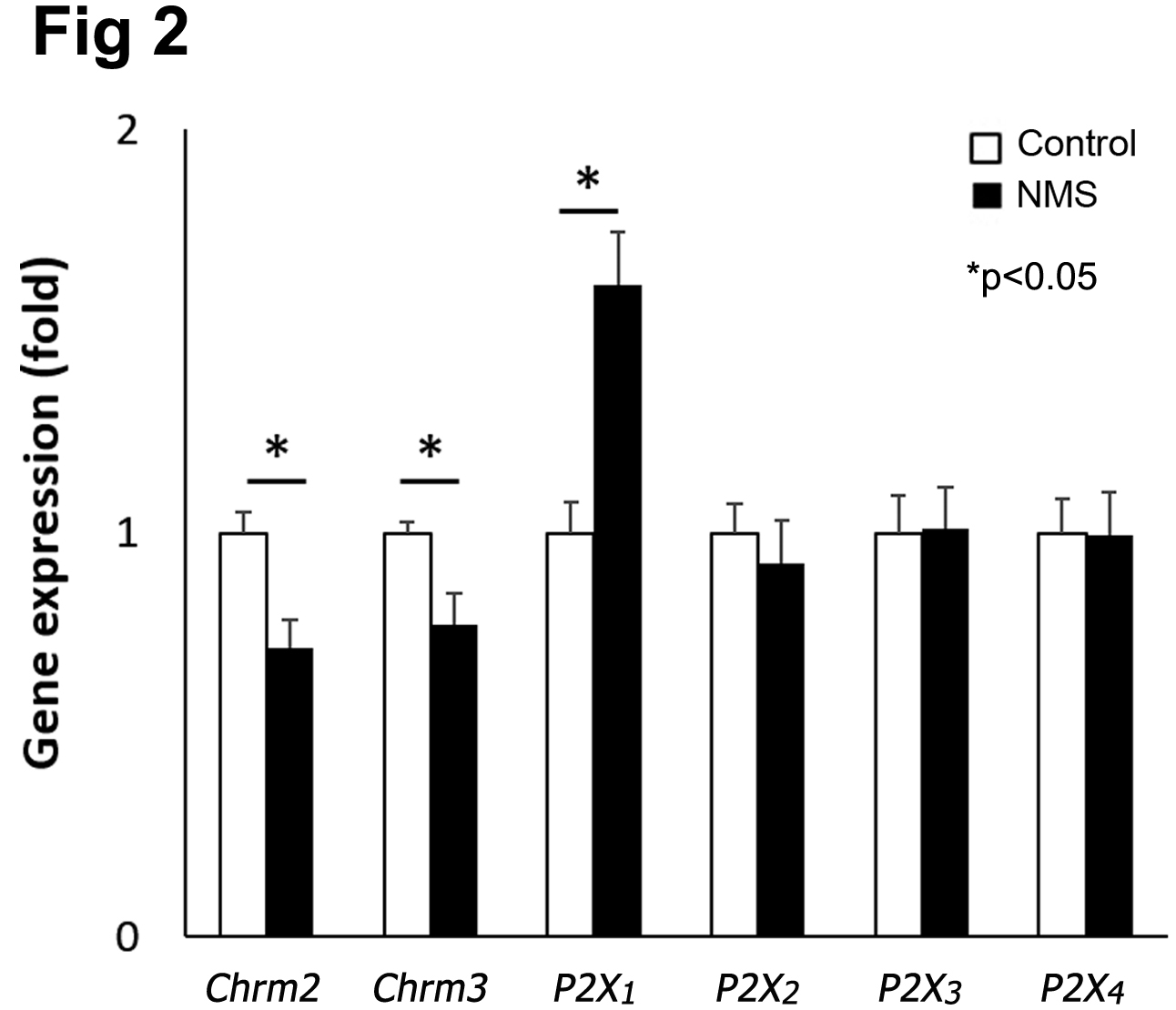
RESULTS: Cystometry showed NMS mice had significantly reduced bladder capacity (106 ± 10 vs. 151 ± 10 ul, p<0.01) and increased non-void contractions (2.9 ± 0.4 vs. 0.7 ± 0.1, p<0.05) compared to control group. NMS mice showed a significant decrease in detrusor contractility in response to in response to carbachol (CCh, muscarinic receptor agonist) by 33% but a higher contractility in response to a purinoreceptor agonist (αβ-MeATP) compared to control mice (Fig 1). There was no difference in detrusor contractility in response to electrical field stimulation (EFS) was detected between 2 groups. Molecular study showed a decreased expression of muscarinic receptors (Chrm2 and Chrm3) and elevated level of a purinergic receptor, P2x1 in the bladders from NMS mice (Fig 2). These results demonstrated that bladder dysfunction in early life altered muscarinic and purinergic signaling contributing to detrusor contractility as described in adult patients with lower urinary tract symptoms.
CONCLUSIONS: Our results provide evidence that early life bladder dysfunction can affect the bladder development and alter the molecular mechanisms that control bladder function. Animal models with NMS protocol can be useful tool to study the mechanisms underlying bladder reflex maturation, which could be used in the future studies for the development of novel therapies to treat voiding dysfunction in children. 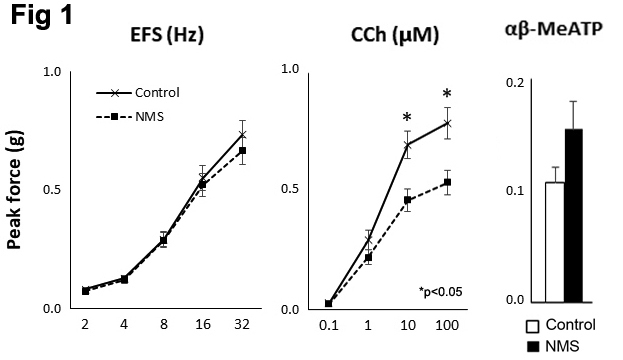
Back to 2018 Program




