Next-Generation Patient Derived Xenografts for Cancer Immunotherapy Responsiveness in Wilms Tumor and Pediatric Rhabdomyosarcoma
Candace F. Granberg, M.D., Patricio C. Gargollo, M.D., Slavic Fedyshyn, MSc, Matthew R. Lowerison, Ph.D., Vidhu B. Joshi, Ph.D., Mario A. Cepeda, Ph.D., Tijani Osumah, M.D., Mohamed Ahmed, M.D., Julie Byrne, CRC, Haidong Dong, M.D., Ph.D., John C. Cheville, M.D., Hon S. Leong, Ph.D..
Mayo Clinic, Rochester, MN, USA.
BACKGROUND: Multi-agent chemotherapy is the cornerstone for treatment of Wilms Tumor (WT) and pediatric rhabdomyosarcoma (RMS) patients. However, a proportion of these patients will exhibit progressive disease during treatment, therefore other therapeutic options such as cancer immunotherapy should be considered at the second line. To direct these studies, we used chick embryos as a patient avatar platform for WT and RMS patients. These studies permit the pre-testing of patient tumor specimens to determine if the tumor infiltrating lymphocytes (TILs) present in the tumor specimen are cytotoxic to tumor cells if treated with checkpoint inhibitor drugs such as nivolumab or pembrolizumab. This avatar platform also permits studies of tumor heterogeneity wherein various regions of the primary tumor or metastases can be individually treated with drug to determine which regions are responsive to the immunotherapy.
METHODS: Primary tumors or metastases from WT (n=1) and RMS patients (n=1) were obtained via nephrectomy and/or metastasectomy. The entire tumor specimen was subdivided into 2-3 mm tumor fragments and all fragments were implanted into the chorioallantoic membrane of Day 9 chick embryos. Chemotherapy (FigA) or cancer immunotherapies (Nivolumab, Pembrolizumab, vehicle)(FigB) were used to treat PDXs to achieve clinically relevant Cmax’s for each drug. Treatment cycles consisted of intravenous injection of drug (50 µL delivery volume) to the surface of the PDX once on Day 13 embryos and again on Day 15 embryos. At endpoint, Day 17 embryos were submitted to high-frequency ultrasound imaging to quantitate changes in tumor volume and tumor vascularity after treatment and compared to saline vehicle control xenografts. Immunohistochemistry was performed to determine the amount of PD-1 and PD-L1 expression within tumors.
RESULTS: Tumor specimens provided by WT and RMS patients revealed a high level of tumor take (>80%). Both sets of PDXs when treated with cancer immunotherapy drugs had xenografts respond with significant decreases in tumor volume and tumor vascularity (FigC-D) as determined by high-frequency ultrasound imaging analysis. While histology did not reveal significant increases in TILs, treated tumors with significant decreases in tumor volume were markedly necrotic. Vehicle treated PDXs were primarily viable via histology and exhibited weak to moderate PD-L1 staining within the tumor.
CONCLUSIONS: The chick embryo model was capable of evaluating a patient’s tumor response to cancer immunotherapy drugs. Mouse models are not capable since the murine stroma will eventually replace original human tumor stroma, making treatment with Nivolumab or Pembrolizumab uninformative. This platform could be used to test patients with diffuse anaplastic WT or RMS to determine if they should receive cancer immunotherapy drugs instead of chemotherapy. 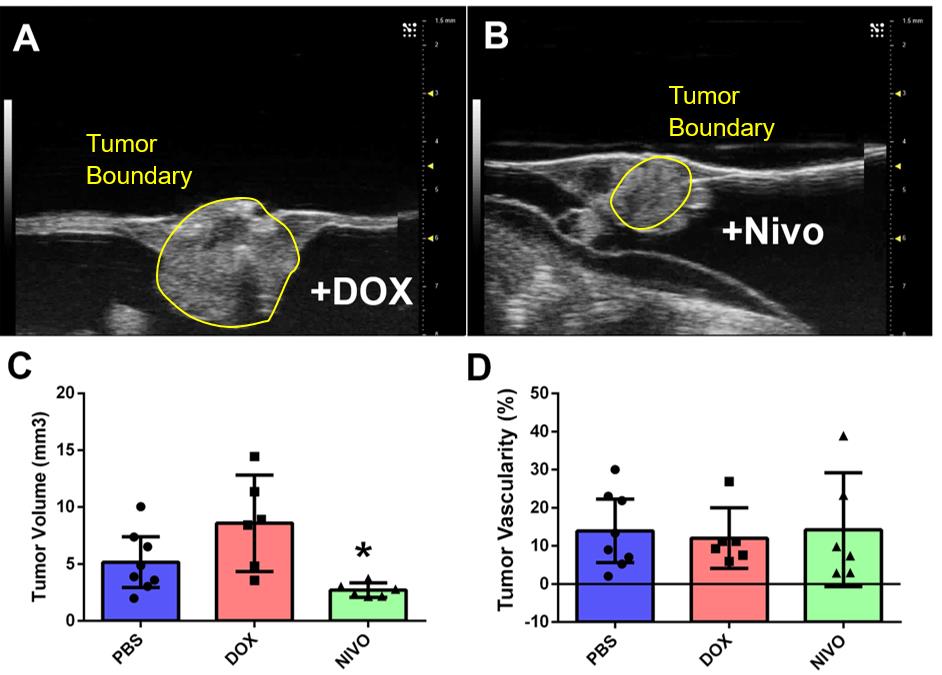
Back to 2018 Program




