Establishing an Institutional Gonadal Tissue Cryopreservation Protocol for Patients with Differences of Sex Development
Courtney J. Harris, MD1, Kristine S. Corkum, MD1, Courtney A. Finlayson, MD2, Monica M. Laronda, PhD3, Molly B. Reimann, BS4, Elizabeth B. Yerkes, MD5, Emilie Johnson, MD, MPH5, Erin E. Rowell, MD1.
1Lurie Children's Hospital, Division of Pediatric Surgery; Northwestern Feinberg School of Medicine, Department of Surgery, Chicago, IL, USA, 2Lurie Children's Hospital, Division of Endocrinology, Chicago, IL, USA, 3Lurie Children's Hospital, Stanley Manne Children's Research Institute; Northwestern Feinberg School of Medicine, Department of Pediatrics, Chicago, IL, USA, 4Lurie Children's Hospital, Division of Pediatric Surgery, Chicago, IL, USA, 5Lurie Children's Hospital, Division of Urology; Northwestern Feinberg School of Medicine, Department of Urology, Chicago, IL, USA.
Background
Advances in fertility preservation (FP) for oncology patients have paved the way for patients with other fertility-threatening treatment regimens, including those with differences of sex development (DSD). Many children with DSD experience subfertility due to abnormal gonadal development, progressive gonadal failure, prophylactic gonadectomy for an increased risk of malignancy, or abnormal hormone production. The ability for patients with DSD to become biological parents is a priority and concern for patients and their families. Although recent studies suggest that germ cells are present in gonads of some patients with DSD, germ cell number appears to decline with age. This presents a potential window of time for patients with DSD to pursue experimental gonadal tissue cryopreservation (GTC). The aim of this study is to describe the development and initial results of an Institutional Review Board (IRB)-approved GTC protocol.
Methods
Prior to 2018, patients with DSD who elected for experimental GTC were enrolled in a protocol designed for pediatric cancer patients. Recognizing important differences between the oncology and DSD populations, our tertiary care pediatric hospital developed an IRB-approved GTC protocol specifically for patients with DSD who are undergoing gonadectomy due to risk of malignancy. Protocol development steps are reported, and a retrospective chart review was conducted to ascertain characteristics of patients who elected to participate.
Results
A multidisciplinary care team provides extensive preoperative counseling with each patient and family. (Figure 1) Pediatric urologists or pediatric surgeons perform gonadectomy for medical indications, for risk of malignancy in gonadal tissue. Once removed, the gonad is bisected with one half sent to pathology to evaluate for gonadal neoplasia and germ cells, and the remaining half is processed for cryopreservation. Given the experimental nature of cryopreserving and future downstream use of this tissue, gonadal tissue is processed so that all tissue is preserved. Tissue is then stored until after pathological analysis and the family has definitively decided whether to cryopreserve the tissue. If neoplasia is noted on pathology, then the cryopreserved gonad half is recalled and sent to anatomic pathology for full examination. Postoperative counseling, and the decision of whether to cryopreserve tissue, is made with the operating surgeon and FP specialist both present. Since the protocol was instituted in 6/2018, four patients have elected to attempt GTC. Diagnoses include ovotesticular DSD, mixed gonadal dysgenesis and Swyer Syndrome (median age at surgery 1.4years [1-13.6years], 3/4 [75%] with germ cells on pathology) and all patients with germ cells present stored tissue.
Conclusions
GTC at the time of gonadectomy is technically feasible for patients with DSD who are at risk for gonadal malignancy. The institutional protocol described represents a template for institutions who wish to offer experimental GTC to patients with DSD who are choosing gonadectomy. Further research topics include determination of patient candidacy, the quality of germ cells, and the optimal timing for GTC in children with DSD. 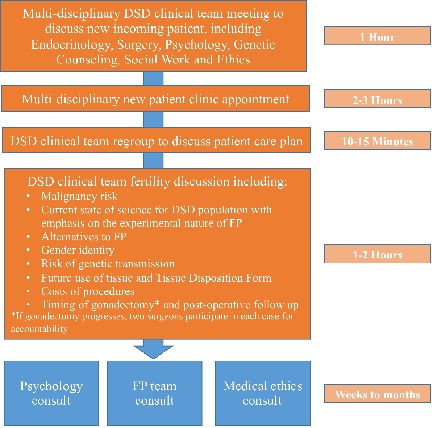
Back to 2019 Abstracts




