QUALITY OF REPORTING FOR PILOT RANDOMIZED CONTROLLED TRIALS IN THE PEDIATRIC UROLOGY LITERATURE - A SYSTEMATIC REVIEW
Melissa McGrath, BASc1, Chen Chen, BSc-C1, Farough Farrokhyar, MPhil, PhD2, Luis H. Braga, MD, PhD1.
1McMaster University, Hamilton, ON, Canada, 2McMaster UNiverity, Hamilton, ON, Canada.
BACKGROUND: The purpose of a pilot study is to examine the feasibility of conducting a larger definitive randomized controlled trial (RCT). The Consolidated Standards of Reporting Trials (CONSORT) statement extension to pilot/feasibility studies was developed in response to the growing number of publications in the literature described as pilot studies, but that did not have real feasibility outcomes or missed important reporting items. The aim of this systematic review was to assess the quality of reporting in pilot RCTs in the Pediatric Urology literature based on their adherence to the CONSORT extension for pilot/feasibility studies.
METHODS: A comprehensive search was conducted through MEDLINEŽ and EMBASEŽ to identify pilot RCTs from 2005-2018. Two reviewers independently performed title and abstract screening as well as full text review, with discrepancies resolved by consensus. Quality appraisal, which was also done in duplicate, was performed using the 17 criteria CONSORT extension checklist (Figure 1). An overall quality of reporting score (OQS) was calculated by dividing the number of checklist items present in each study by the maximum possible score (17) and expressed as a percentage. Studies were then classified as low (<40%), moderate (40-70%) and high OQS (>70%). Mean OQS was compared with the presence or absence of four a priori key methodological factors. Results were considered statistically significant when p<0.05. Data were analyzed using SPSS version 22.0.
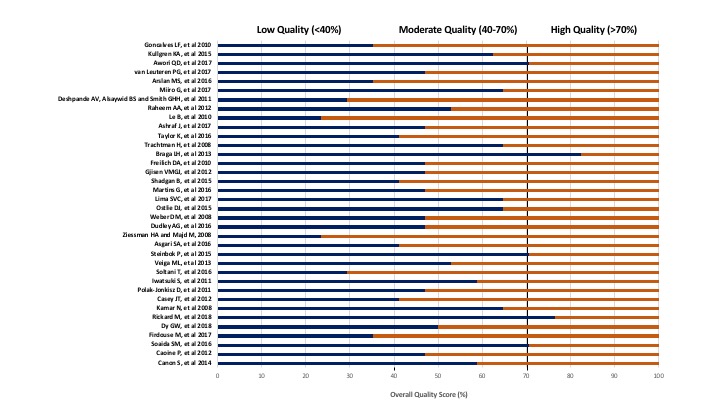
RESULTS: Of the 1347 titles screened, 36 studies were included. The mean OQS was 51%+15% with 7 articles (19%) identified as low quality (score <40%), 24 (67%) as moderate (40-70%) and 5 (14%) as high quality (>70%) (Figure 2). Mean OQS of pilot RCTs published between 201619 (n=30) vs those reported before 2010 (n=6) was not significantly different (45+18.5% vs. 52+14%, p=0.). However, mean OQS of pilot RCTs with a biostatistician support (n=4) was significantly higher than those without support (n=32) (69+10% vs. 49+14%, p=0.01). Studies that described the method of randomization had significantly higher OQS (n=11) vs. those that did not (n=25) (63+11% vs. 45+13%, p=<0.01), as well as studies which included sample size justification (n=6) vs. those that did not (n=30) (69+11% vs. 47+13% p=<0.01).
CONCLUSION: The mean OQS of pilot studies in pediatric urology was suboptimal (51%). Key variables that were significantly associated with a higher OQS were biostatistician support, sample size calculation and method of randomization. Therefore, adopting the CONSORT extension checklist as a prerequisite for submission of studies identified as 'pilot' may improve the reporting and transparency of pilot studies, leading ultimately to improved implementation of future RCTs. 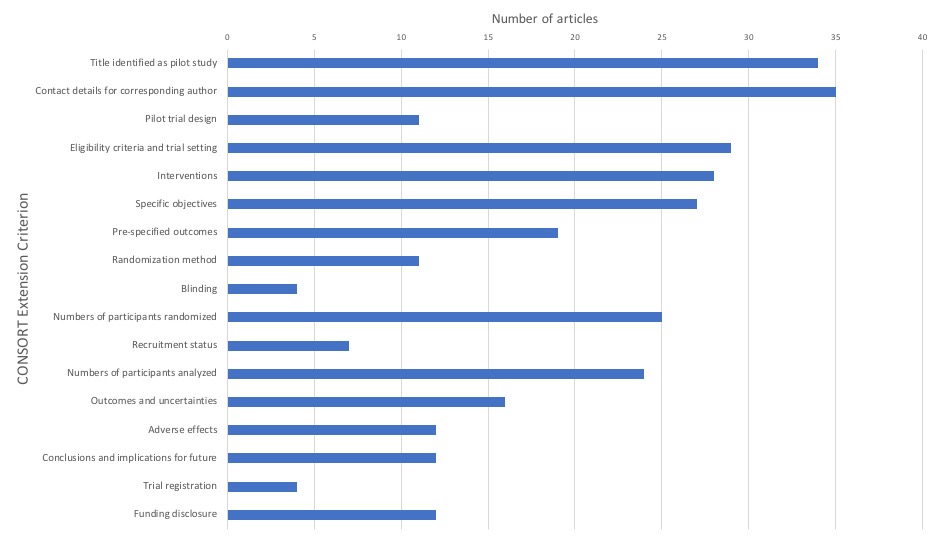
Back to 2019 Abstracts




