Modeling cyp21a2 dependent steroidogenesis in zebrafish for application in Congenital Adrenal Hyperplasia
Jun Yao, Ph.D.1, Kiersten Craig, M.D., M.S.E.1, Dix Poppas, M.D., F.A.C.S.2, Yariv Houvras, M.D., Ph.D.1.
1Weill Cornell/ New York Presbyterian, New York, NY, USA, 2The Comprehensive Center for Congenital Adrenal Hyperplasia, Komansky Children's Hospital, New York-Presbyterian Hospital-Weill Cornell Medicine, New York, NY, USA.
BACKGROUND: Congenital adrenal hyperplasia (CAH) is an autosomal recessive disorder caused by loss of function mutations or deletions affecting the cytochrome P450 family 21 (CYP21A2) gene. Patients with CAH experience aldosterone and cortisol deficiency and may also have adrenal androgen excess. Current management of patients with CAH requires life-long exogenous steroid treatment, mineralocorticoid replacement, and often surgery. There is a need to understand the disease mechanism and identify novel drugs for CAH patients. Zebrafish interrenal steroidogenic cells and the adrenal biosynthesis pathway are evolutionally conserved with humans, including a CYP21A2 ortholog, making it a potentially useful model to study CAH.
METHODS: Development of a cyp21a2 reporter line by genome editing: We developed a fluorescently labeled cyp21a2 reporter zebrafish using CRISPR/Cas9 genome editing. Using homology-directed repair we targeted the cyp21a2 locus to create a fusion gene with mScarlet. We identified founder zebrafish that transmit this allele through the germline.
Building a CAH Model with cyp21a2 Disruption: A cyp21a2 mutant zebrafish was created using CRISPR/Cas9 to target exon 6 of the zebrafish cyp21a2 gene. The genotype of wild-type, heterozygous, and homozygous mutant zebrafish, was confirmed usingrestriction fragment length polymorphism assay (BfaI), and polymerase chain reaction (PCR) followed by Sanger sequencing. Steroidogenic deficiency was assessed using quantitative PCR (qPCR) and cortisol enzyme-linked immunosorbent assay (ELISA) assay.
RESULTS: We created a cyp21a2-2a-mScarlet reporter zebrafish, allowing us to study the temporal and spatial expression of cyp21a2 throughout development (Fig 1A&B). Through transcriptome analysis and real-time in vivo imaging of zebrafish interrenal steroidogenic cells, a specific gene expression signature was identified, providing new insights into the CAH disease mechanism which may lead to the discovery of potential drug targets. The cyp21a2 mutant line has a 7-bp deletion (cyp21a27∆/7∆) which caused a coding frameshift with an early termination. The cyp21a27∆/7∆ homozygous mutant larvae had dramatically reduced concentration of cortisol at 6dpf (p<0.05, Fig 1C). The expression of adrenal specific markers (starand cyp11c1)were significantly higher in cyp21a27∆/7∆ homozygous mutant than their sibling wild-type fish. Additionally, pomca (an ACTH precursor gene) was significantly higher in cyp21a27∆/7∆ homozygous mutant, indicating feedback response from the hypothalamic-pituitary-interrenal axis (p<0.001, Fig 1D).
CONCLUSIONS: We developed a real-time in vivo cyp21a2reporter model and a CAH mutant zebrafish that can be used to provide new insights into CAH. Future studies using chemical-genetic approaches in these models may translate to a novel treatment strategies for patients with CAH. 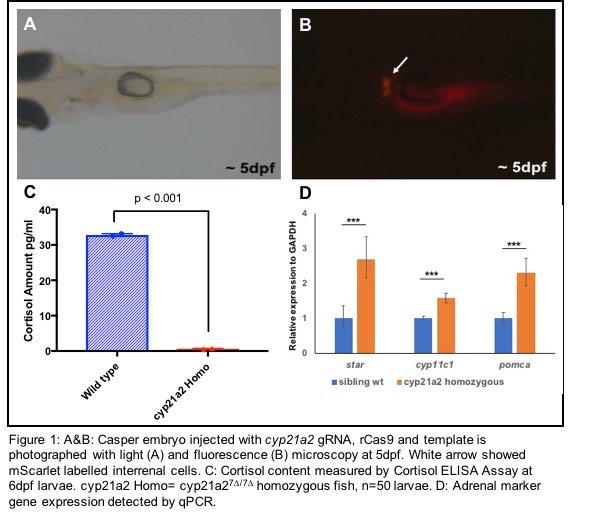
Back to 2019 Abstracts




