Prolyl Hydroxylase Inhibitors: A Novel Drug Class that Protects the Bladder Against Cyclophosphamide-Induced Cystitis
Ching Man Carmen Tong, DO1, Shuvro De, MD1, Abby S. Taylor, MD1, Stacy T. Tanaka, MD1, John C. Thomas, MD1, John C. Pope, IV, MD1, Mark C. Adams, MD1, John W. Brock, III, MD1, Volker H. Haase, MD2, Douglass B. Clayton, MD1.
1Division of Pediatric Urology, Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN, USA, 2Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Introduction
Prolyl hydroxylase inhibitors (PHI) are a novel class of oral compounds currently in phase III clinical trials for treating anemia of chronic kidney disease. PHI exert cytoprotective effects by activating the hypoxia-inducible factor (HIF) pathway and its myriad downstream targets. In a mouse model of cyclophosphamide (CYP) cystitis, we previously showed that treatment with the PHI, dimethyloxalyglycine (DMOG), protects the bladder from CYP injury by modulation of HIF pathway. We hypothesize that a promising new oral PHI, Bay 85-3934, will provide cytoprotection in a mouse model of CYP cystitis.
Methods
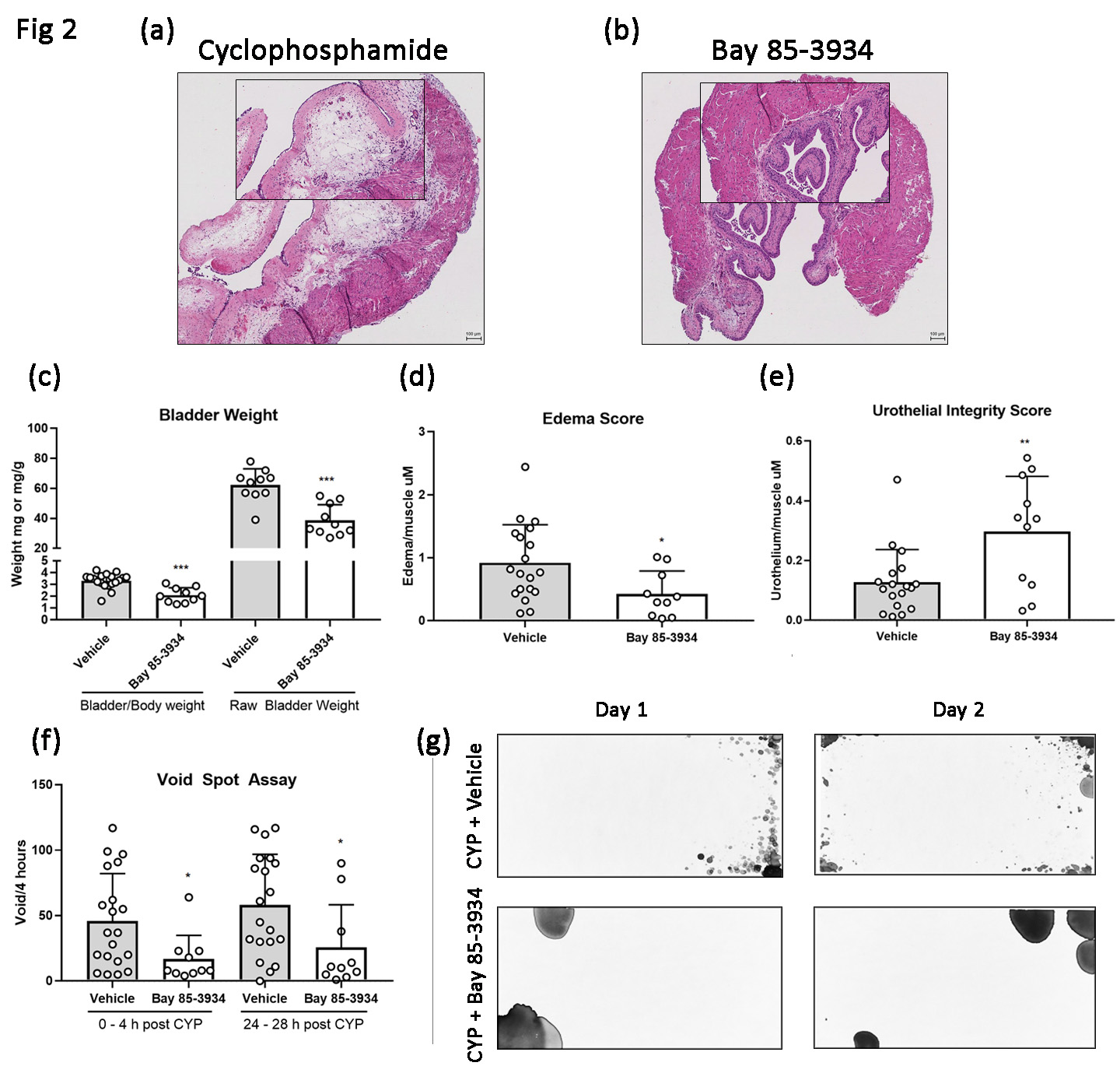
All experiments were approved by our institutional animal care and use committee. To induce cystitis, 8 – 10 week old male C57BL/6 mice received 300 mg/kg of intraperitoneal (IP) CYP. To study PHI-mediated cytoprotection, experimental mice received either a single dose of IP DMOG (16 mg/animal) at the time of CYP administration or oral gavage of Bay 85-3934 (10 mg/kg) given in 3 doses 48 hours, 24 hours and 6 hours prior to CYP administration. IP saline served as vehicle control for DMOG experiments while oral 10% ethanol/ 90% sunflower oil served as vehicle control for Bay 85-3934. Animals were euthanized 48 hours after CYP administration. Measurements of inflammatory injury included ex-vivo bladder weight, bladder weight to body weight ratio, and high-throughput histologic analysis of h&e staining using a novel injury scoring system to assess urothelial integrity and transmural edema. Mouse voiding function was analyzed using Voided Spot Assays (VSA) collected over 4 hours at hour 0 and 24 hours after CYP. All VSA images were uniformly analyzed using Void Whizzard software for imageJ. Groups were compared with Mann Whitney U tests with significance set at p <0.05.
Results
Following CYP administration, we noted reduced inflammatory changes in DMOG-treated (fig 1b) and Bay 85-3934 treated animals (fig 2b) as compared to vehicle controls (fig 1a, 2a). We also observed statistically significant reductions in ex-vivo bladder weight in the DMOG and Bay 85-3934 groups as compared to vehicle (fig 1c and 2c). Likewise, scores of transmural edema and scores of urothelial integrity were improved in the experimental groups (fig 1d and 1e, 2d and 2e, respectively). In addition, animals treated with either DMOG or Bay 85-3934 voided less frequently compared to controls (fig 1f and 2f, respectively). Figures 1g and 2g demonstrate VSA voiding patterns from mice treated with vehicle, DMOG, and Bay 85-3934, respectively.
Conclusion
Our results demonstrate a potential role for oral PHI treatment in patients with CYP-induced hemorrhagic cystitis. This work also emphasizes the role of acute HIF pathway activation in modulating inflammatory responses within the bladder. Future work will include the use of transgenic mouse models to better understand the mechanisms that underpin this therapeutic effect. 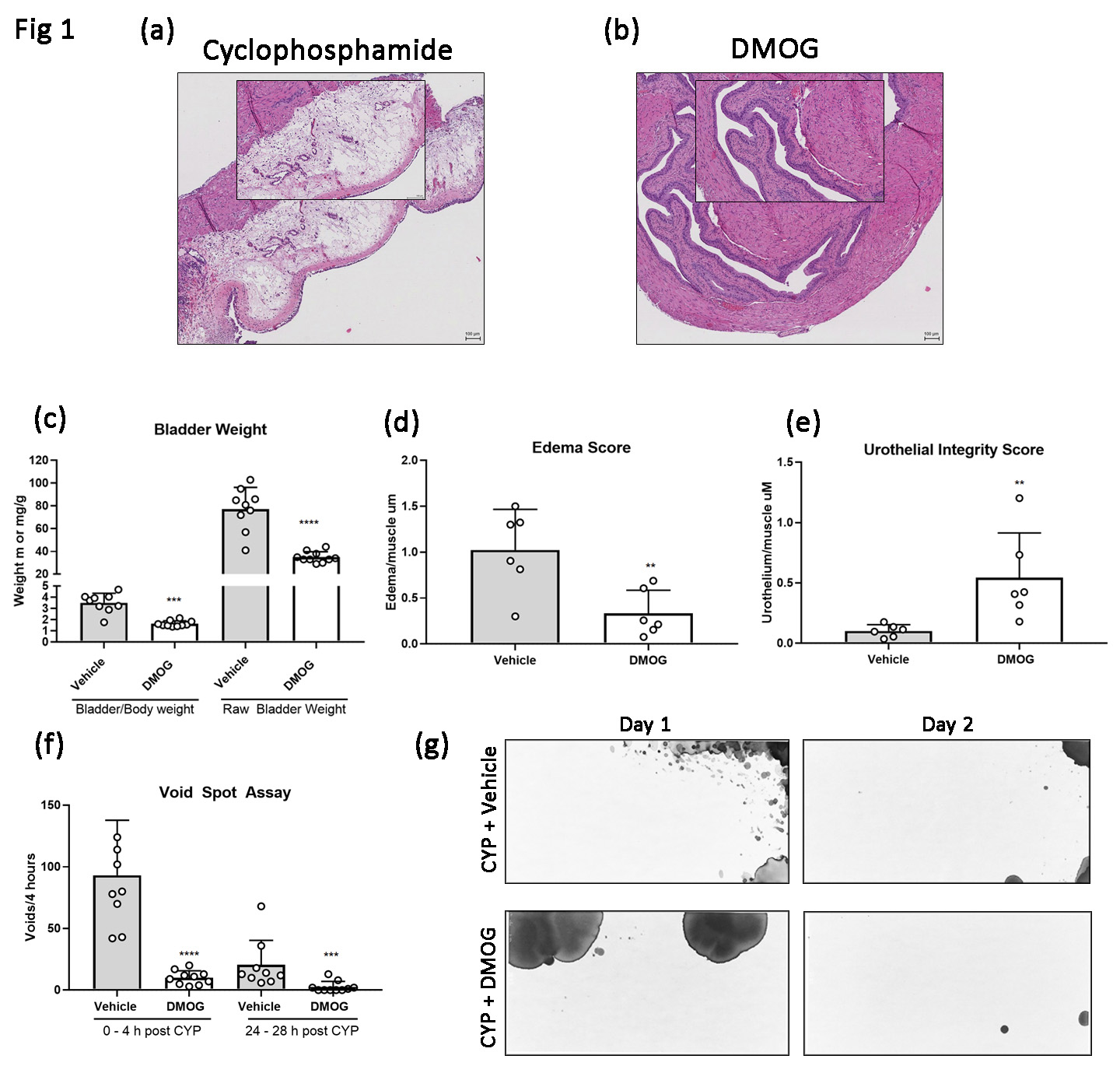
Back to 2019 Abstracts




