H-IPSE, a Parasite-Derived Candidate Drug for Bladder Pain, Localizes within Cells Besides the Urothelium
Olivia Lamanna, B.S. in Biology1, Evaristus Mbanefo, PhD1, Kenji Ishida, PhD1, Franco Falcone, PhD2, Theodore Jardetzky, PhD3, Luke Pennington, MD/PhD Candidate3, Michael Hsieh, MD/PhD4.
1Biomedical Research Institute, Rockville, MD, USA, 2University of Nottingham, Nottingham, United Kingdom, 3Stanford University, Stanford, CA, USA, 4Children's National Medical Center, Washington, DC, USA.
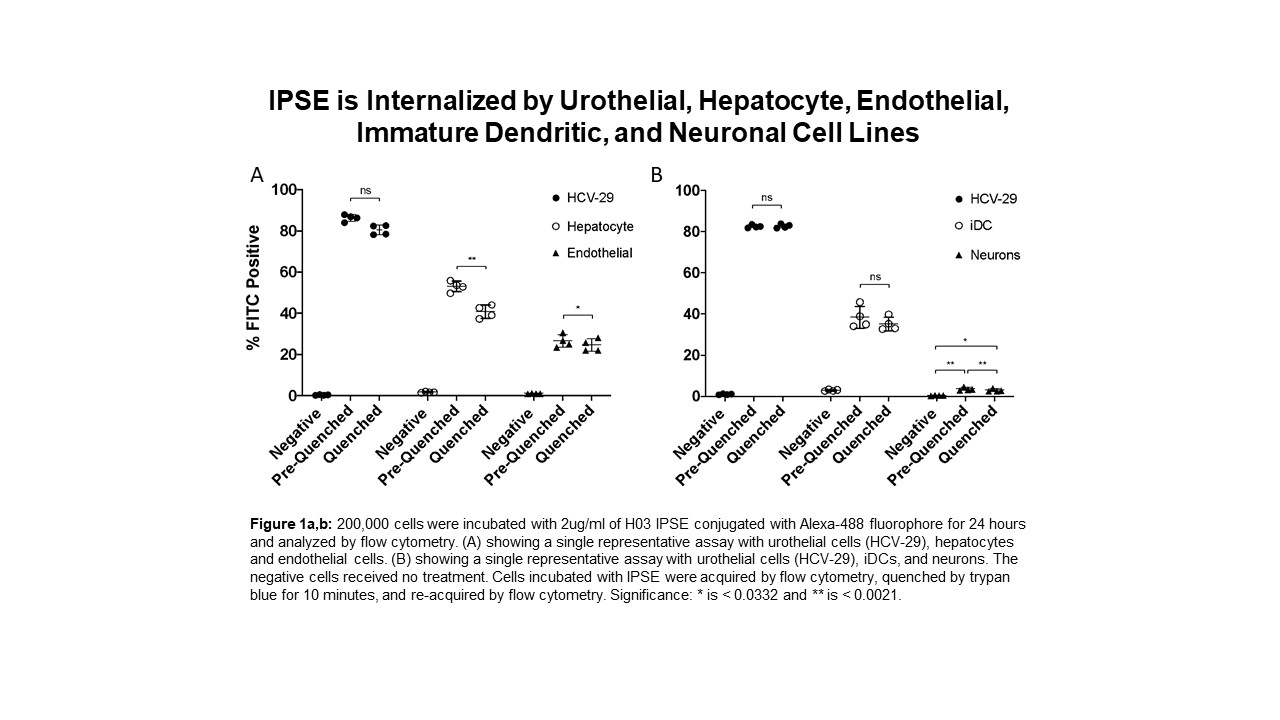
Our lab has demonstrated that H-IPSE, the Schistosoma haematobium ortholog of the IL-4 Inducing Principle from Schistosoma mansoni Eggs, is therapeutic in ifosfamide-induced hemorrhagic cystitis through the downregulation of pro-inflammatory pathways. We also have data suggesting IPSE alleviates resiniferatoxin (capsaicin receptor)-induced bladder pain. IPSE has been shown to interact with IgE on the surface of basophils to induce IL-4 secretion, trigger Breg cell activation, sequester chemokines, and, as an infiltrin, translocates into host cell nuclei to alter host transcription. We have previously shown that nuclear translocation-deficient mutants of H-IPSE lose their therapeutic effects, suggesting that intracellular localization of H-IPSE is particularly important for its activity. Our objective was to compare the magnitude of IPSE's intracellular localization within urothelial cells to that of other cell types to determine what portion of IPSE's therapeutic effects, if any, are exerted on non-bladder specific cells within the bladder. H03, an H-IPSE ortholog, was conjugated to Alexa-488 fluorophore and the degree of labeling was confirmed by spectrophotometry. H03 was incubated with multiple cell lines including urothelial cells (HCV-29), endothelial cells, immature dendritic cells, hepatocytes, and neurons. Flow cytometry was used to quantify the percentage of cells that were H03 positive. Trypan blue quenching of extracellular Alexa-488 signal was used to measure intracellular IPSE signal. At 2 ug/ml, H03 was not internalized until 6 hours of incubation with cells, and a peak signal was observed at 24 hrs. When urothelial, endothelial, hepatocyte, immature dendritic cells, and neuronal cell lines were incubated with H03 for 24 hours, all cell lines demonstrated significant IPSE internalization, however, urothelial cells internalized IPSE most efficiently (figure 1a,b). IPSE internalization by neurons was minimal at about 6.7% of the population, which was tenfold less than IPSE's internalization by urothelial cells (figure 1b). Neurons, endothelial cells and hepatocytes had featured subpopulations in which IPSE was bound to the cell surface as well as internalized. This was not the case with urothelial and immature dendritic cells as IPSE was predominantly internalized. H-IPSE can be internalized by urothelial, endothelial, hepatocyte, iDC, and neuronal cell lines, suggesting that IPSE's therapeutic function is not isolated to the urothelium or bladder-specific cells. However, the magnitude of IPSE internalization by non-urothelial cells is significantly less compared to urothelial cells, especially neurons. We hypothesized that IPSE could be acting through the capsaicin receptor on nociceptive neurons to alleviate pain. However, our findings indicate that IPSE interacts with neurons minimally and is most likely inducing its effects through an alternative pathway. Additional research on IPSE's extracellular targets and internalization mechanism is underway to provide insight on why IPSE favors the urothelium and how other cell types could contribute to its functions.
Back to 2019 Abstracts




