Early Animal Feasibility Studies of the Vortex shunt in fetal ovine models for lower urinary tract obstruction (LUTO) and pleural effusion
Kunj R. Sheth, MD1, Enrico Danzer, MD2, Marianna Scuglia, MD3, Tomohiro Arai, MD3, Eric Johnson, MS1, James K. Wall, MD1, Katerina Zapletalova, MD4, Wasinee Tianthong, MD4, David Basurto, MD, PhD3, Roland Devlieger, MD, PhD3, Francesca M. Russo, MD, PhD3, Jan Deprest, MD, PhD3, Yair J. Blumefeld, MD1.
1Stanford School of Medicine, Stanford, CA, USA, 2Memorial Sloan Kettering Cancer Center, New York City, NY, USA, 3University Hospitals Katholieke Universiteit (KU) Leuven, Leuven, Belgium, 4, University Hospitals Katholieke Universiteit (KU) Leuven, Leuven, Belgium.
Background: The Vortex shunt is a novel shunt for the prenatal treatment of fetal lower urinary tract obstruction (LUTO), as well as fetal macrocystic lung lesions and pleural effusions. The key design elements of the Vortex shunt are: 1) [ED1] Nitinol braided anchors to provide robust, atraumatic fixation, and redundant infusion ports, 2) Nitinol coil reinforced conduit with Polyurethane coil covering for kink resistance and extensibility, 3) an integrated one-way duckbill valve to allow intermittent bladder, 4) Echogenic visualization aids for ultrasound-guided delivery, and 5) a 7Fr delivery system design that allows shunt repositioning and recapture. The current large animal feasibility study aims to evaluate shunt deployment, drainage and early (within 24 hours) dislodgement risk in a fetal ovine model.
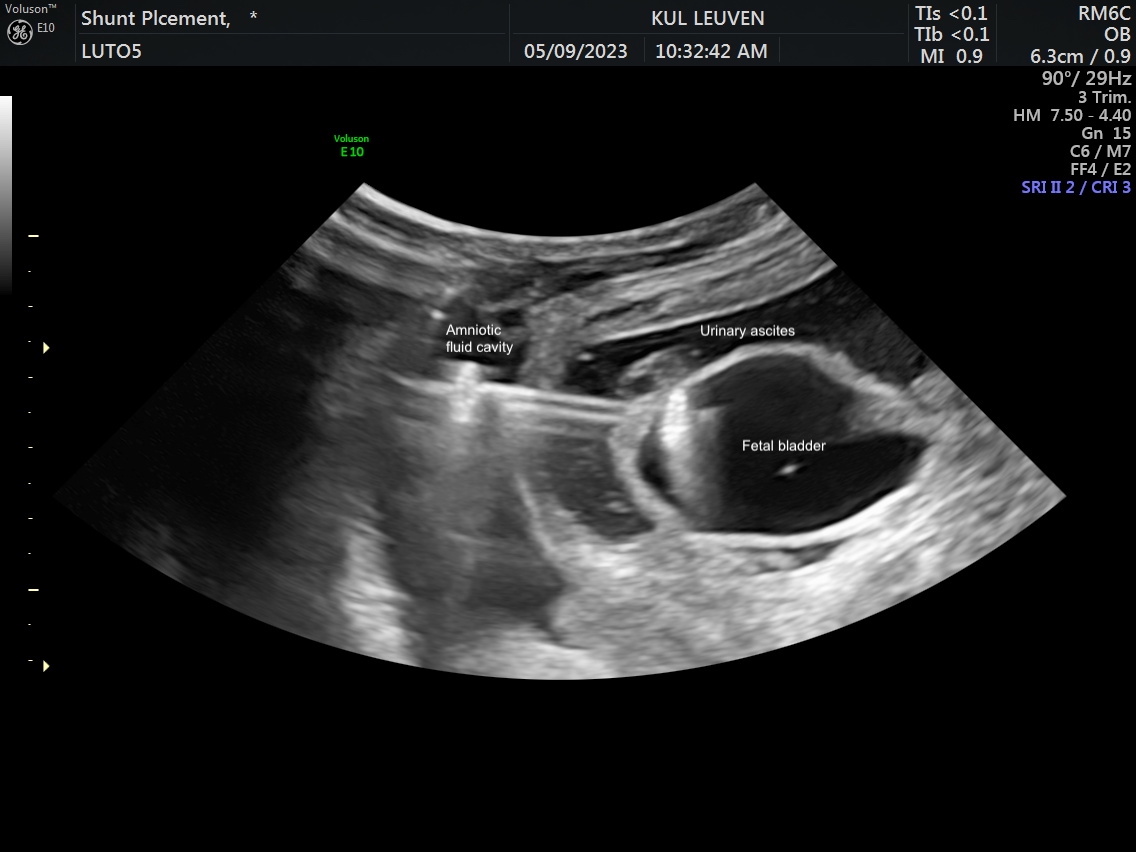
Methods: An acute pleural effusion model was developed by injecting saline into the fetal pleural space. Percutaneous thoracoamniotic shunt deployment was pursued in four fetuses at mid-gestation followed by immediate euthanasia and necropsy. The LUTO model was created in eight ewes at a median of 70 days (range, 69-72) gestation by ligating both the urethra and urachus. Severe LUTO with ultrasound features of megacystis, hydronephrosis, urinary ascites, and significant oligohydramnios/anhydramnios developed in 5 fetuses of which 4 survived. At a median of 98 days (range, 97-99), these 4 LUTO fetuses underwent Vortex shunt placement (2 non-valve shunts and 2 valve). Twenty-four hours post-deployment, shunt location was confirmed on fetal ultrasound and then on necropsy.
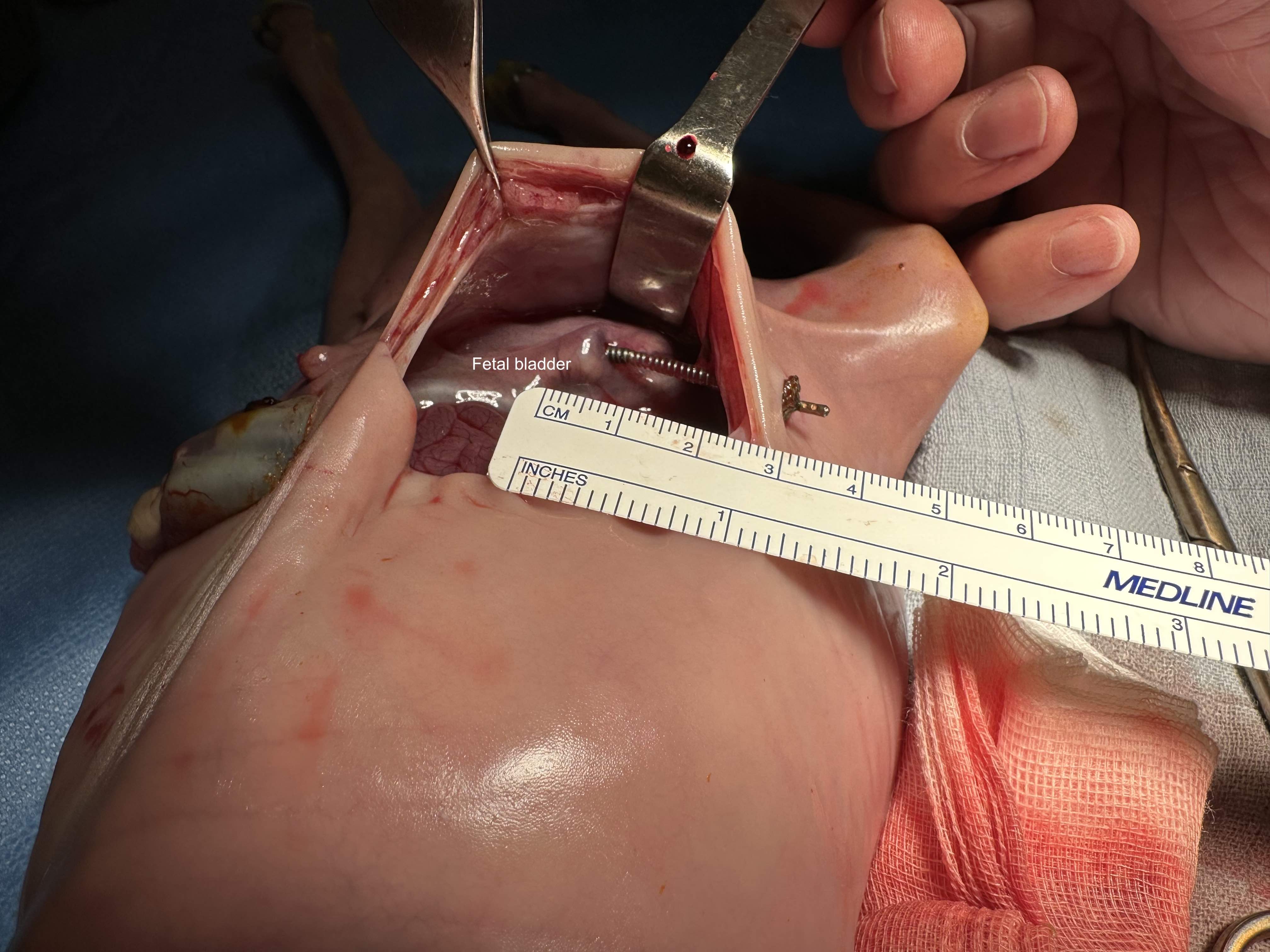
Results: All 4 thoracoamniotic shunt placements were successful placed with complete drainage into the amniotic space within minutes of deployment. For the 4 LUTO shunts, deployment was successful with the proximal anchor in the bladder and the distal anchor at the fetal abdominal wall within the amniotic sac (Figure 1). No dislodgement or kinking of the shunts were noted at 24 hours post-deployment on ultrasound with substantial improvement in oligohydramnios/anhydramnios. For the two non-valve shunts, the bladder was fully decompressed at 24 hours. For the two valve shunts, the bladder was partially decompressed at 24 hours with substantial decrease in bladder volume and urinary ascites. Correct shunt position was confirmed for all shunts on necropsy, and, all shunts were successfully removed through re-sheathing of the shunt. No damage to any surrounding organs was noted along the trajectory of the shunt deployment path (Figure 2).
Conclusion: Initial fetal ovine feasibility studies show successful deployment at mid-gestation of the Vortex shunt with no dislodgement at 24 hours. The valve shunt shows promise with clear improvement in urinary tract dilation after deployment without continuous bladder drainage. With the successful feasibility studies, we plan to proceed with long-term survival studies, from initial shunt placement in the ovine LUTO model until term.
Back to 2023 Abstracts
